Chapter 13
Female Hormonal Contraceptives
A functional account about progestogens, androgens
and oestrogens has already been given in Chapter 5. It included the metabolic,
morphological and endocrine effects of these three different steroids. It will
be an impossible task to write about all brands of the hormonal contraceptives
available worldwide. Many good brands are licensed in certain but not other
countries. Accordingly, this chapter is not meant to give a detailed account of
such different variations, or the instructions on how to use different
contraceptives. It is written with the following objectives in mind, avoiding
repetition of previously discussed issues:
- A broad
account of the different types of female hormonal contraceptives and their
mode of action;
- The
non-contraceptive benefits of hormonal contraceptives;
- Use of
hormonal contraceptives by patients with known medical problems;
- Complications
and risks of hormonal contraceptives;
- Emergency
contraception;
- Perimenopausal
contraception.
Types of
hormonal contraceptives and their mode of action
Despite the great advances in the field of family planning
over the last 30 years, oestrogens and progestogens remained the cornerstones
for hormonal contraceptives, though newer means for their delivery have been
introduced. Beside the oral route, transdermal, transvaginal and intrauterine
routes have been used effectively, and with great acceptance by women.
Progestogens played the major part in hormonal
contraception, and are mostly derived from three parent steroid molecules which
are estranes, gonanes and pregnanes. These molecules differ in relation to
their half lives, and their antioestrogenic effect. Furthermore, gonanes have
17 carbon atoms, whereas estranes have 18. The range of progestogens used
includes norethindrone, norethindrone acetate, ethynodiol diacetate,
norgestrel, levonorgestrel, norethynodrel, desogestrel, norgestimate, and
gestodene. The last three are derived from the gonane molecule, and are known
as the third generation progestins. Other gonane derivatives include norgestrel
and levonorgestrel. Estranes include norethindrone, norethindrone acetate,
ethynodiol diacetate, and lynestrenol. Pregnanes include medroxyprogesterone acetate and megestrol acetate, and they have no
androgenic activity. They are used in the injectable forms of hormonal
contraceptives. The sequence of ascending androgenicity of the different
progestogens used is: ethynodiol diacetate, norethindrone, norethindrone
acetate, norgestimate and desogestrel, in that order. Levonorgestrel and
gestodene have the highest androgenicity in all groups. This information is of
great value when selecting a specific brand of contraceptive pill, especially
when dealing with hyperandrogenic patients. Furthermore, progestogens of lower
androgenic capability are less likely to oppose the oestrogen induced hepatic
production of sex hormone binding globulin and high density lipoprotein (HDL).
A new spironolactone analogue (drospirenone) which has
anti-mineralocorticoid, anti-androgenic and progestational activities has been used in oral
contraceptive pills, mainly for women with polycystic ovary syndrome and
premenstrual dysphoric disorder.
In contrast to the long list
of progestins, only two oestrogens are used in the majority of combined
hormonal contraceptives; ethinyl oestradiol and mestranol. Ethinyl oestradiol
is pharmacologically active, whereas mestranol has to be converted into
oestradiol first before gaining biologically activity. Oral contraceptives
currently in the market contain 20 - 35 micrograms of oestrogen. Pills with 50 µg
ethinyl oestradiol are still available, but are used for the management of
certain gynaecological problems when high doses of oestrogen are needed, rather
than for regular contraceptive purposes. Unlike all the
other routes, orally taken ethinyl oestradiol is
absorbed rapidly from the intestines and undergoes rapid metabolism in the
liver during the first hepatic pass which reduces its biological efficacy by almost
40%. It has plasma half life of 10-27 hours, with a
longer half-life in tissues, such as the endometrium. This first hepatic pass affects different liver functions, including
increased production of clotting factors, sex hormone binding globulin,
transcortin, thyroid binding
globulins, as well as changes in the lipid profile. This may have direct
positive or negative effects on different individuals, depending on their own
circumstances, such as patients with hypothyroidism on thyroxine replacement
therapy. Such hepatic first pass does not occur with the transdermal route,
which can be of benefit especially for patients at risk of thromboembolism.
Hormonal contraceptives can
be used in different forms:
Oral contraceptives
Oral contraceptives can be used either in a combined
oestrogen and progestogen form, or as progestogens only pills. Regular brands
of the combined form have 21 pills, either in monophasic, biphasic or triphasic
combinations. Monophasic pills have the oestrogen and progestogen in a fixed
dose in the same pill for the whole 21 days. Biphasic and triphasic brands have
oestrogen and progestogen pills in two or three different doses to be taken in
sequence respectively. The idea behind these different combinations was to
reduce the total amount of hormones used, and to simulate the variations in
oestrogen and progesterone which occur during a natural ovulatory cycle. Newer
brands of oral contraceptives have been manufactured to reduce the hormone free
period by increasing the number of pills to be taken each cycle. Brands with 24
or 26 instead of 21 pills have been marketed. The main objective behind this
regimen was to have better control of the bleeding episodes, and to reduce the
premenstrual symptoms suffered by the patients during the hormone free period.
Such brands are sold in the United States, but are not licensed yet in the
United Kingdom. An extended protocol to use oral contraceptive pills for 84
days before having a withdrawal bleeding proved useful for patients with
endometriosis and severe premenstrual syndrome. Seasonale
(Duramed Pharmaceuticals, Inc) is a brand which contains 30 µg ethinyl
oestradiol and 150 µg levonorgestrel. Yet again, despite the wide increase in
the number of brands of combined hormonal contraceptives currently available,
suppression of ovulation and local uterine changes remained to be the most
important modes of action. These are affected through the synergistic effects
of both oestrogen and progestogen. Nonetheless, in most cases the contraceptive
efficacy is linked to the progestogen part, with oestrogen controlling regular
bleeding.
Unlike oestrogens, progestogens can be used
separately to provide adequate contraception. Progestogens only pills have been
available for a long time, and are sometimes known as mini pills. They are
mainly used when oestrogen is contraindicated. This is especially so in cases
at risk of cardiovascular diseases and venous thromboembolism. They are also a
good choice for lactating women requiring contraception, as they do not affect
milk production, and do not influence the infant’s growth or development.
Unlike the combined forms, they should be used continuously and at the same
time every day without a break. Different brands are available and their main
contraceptive effects have been related to the following points:
· They increase cervical mucus
viscosity which reduces transcervical sperm migration into the uterine cavity.
This effect is maximal four hours after intake. The pills should be taken at
the same time every day, as serum levels fall to baseline levels within 24
hours after ingestion. Other precautions should be taken for 48 hours if taking
a pill is delayed for more than 3 hours.
· They can inhibit activation
of the enzymes necessary for sperm capacitation which is required for ovum
penetration.
· They slow ovum transport
through the fallopian tubes, which may increase the risk of tubal pregnancies.
· They reduce embryos
implantation due to their progestational histological effects on the
endometrium. Progestogens deplete their own receptors and oestrogen receptors
in the endometrium. Biochemically, progestogens reduce the production of
glycogen in the endometrium, and accordingly reduce the ability of the
blastocyst to survive in the uterine cavity.
· Small doses of progestogens
as used in the progestogen only pills do not usually inhibit ovulation, and
many women continue to ovulate normally. Occasionally, only the LH surge is affected despite growth of
the follicles to a mature size. This explains the increased risk of functional
ovarian cysts seen in patients using
progestogen only pills.
Injectable
contraceptives
The two main injectable forms are
depo-medroxyprogesterone acetate (depoprovera) and combined injectable
contraceptives (CICs), both being long acting contraceptives.
Depoprovera can be used in a dose of 150 mg every 3 months. Its blood level
peaks approximately 10 days after drug administration, and sustain high blood
levels capable of inhibiting ovulation by direct action at the hypothalamus and
pituitary gland. It also has a strong progestational effect at the level of the
endometrium. Amenorrhoea is common after the third dose in almost 50% of the
users, with the majority of the remaining group having irregular bleeding
episodes (1). After one year of use, about 75%
of the users will be amenorrhoeic. It may take up to 200 days for the drug to
clear off the circulation following a single injection, as documented in the
Depoprovera Product Monograph (2). This slow decline in the level of serum
progestogen is responsible for the long period of amenorrhoea and delayed
return of fertility. It took 9 months after the last injection on average for
women to conceive as reported in the same Monograph (2).
In comparison to intrauterine contraceptive devices (IUCD) and oral
contraceptives, 92% of women conceived within 2 years after discontinuing
depoprovera, 93% after IUCD
and 95% after stopping the pill (2). Partial
reactivation of the hypothalamo-pituitary-ovarian axis may allow basic folliculogenesis to restart
leading to development of mature follicles, with failure of the LH surge mechanism. This will prevent the final act of
ovulation, or may lead to abnormal ovulation and dysfunctional uterine bleeding. Being a strong
antioestrogen, it has been associated with the development of osteoporosis. This effect has been quantified
by the Product Monograph alluded to before (2), which reported 5-6% reduction in the spine and hip
bone mass density after 5 years of depoprovera use. This decline was more
pronounced during the first two years of medication. This effect was not
carried through during the postmenopausal years as shown by a World Health
Organisation study, which showed similar bone mass density in women who did or
did not use depoprovera (3). Furthermore,
young women in their teenage years are more likely to regain their bone mass
density within 12 months after stopping medication. An advantage of depoprovera
is that it has no androgenic effect. Furthermore, it does not affect the level
of triglycerides or total cholesterol (4), and has no significant detrimental effect on the liver
function, coagulation factors, fibrinolysis, or blood pressure.
Alterations in carbohydrate metabolism similar to those imposed by the oral
contraceptive pill can follow depoprovera administration, but to a lesser
extent and with minor clinical significance (5). More information about depoprovera has been
given in Chapter 5. Another injectable progestogen is Noristerat, which is made
of 200 mg norethisterone enanthate. It should be given by deep intramuscular
injection on the fifth day of the cycle for short term effective contraception,
when patient’s compliance is not guaranteed regarding the pill. It can be
repeated after eight weeks if necessary. It has similar mode of action to
depoprovera, but menstrual irregularities are less common.
Combined injectable contraceptives (CICs) contain
both oestrogen and progestogen, and are given by monthly intramuscular
injections. Their mode of action is similar to that of the combined oral
contraceptive pill, with similar range of side effects and contraindications,
but without the first hepatic pass. They offer better cycle control
and quicker return of fertility after suspending medication than the
progestogen only injections. There are three main types of
CICs including Cyclofem, Mesigyna and Deladroxate. Cyclofem (cyclo-provera)
contains 25 mg depo-medroxyprogesterone acetate, plus 5 mg
oestradiol cypionate. Mesigyna is made up of 5 mg oestradiol
valerate and 50 mg norethisterone enanthate. Deladroxate contains 10 mg
of oestradiol enanthate and 150 mg dihydroxyprogesterone acetophenide. The
first dose should be given within the first 5 days of menstruation, and repeat
injections administered every 28 days. They offer an error margin of 5 days
only. Because of their depo effect, ovulation and fertility usually return 2-3
months after the last administered dose (6). A
recent Cochrane database systemic review showed that more women using CICs had
normal bleeding, and
fewer of them stopped using them because of bleeding reasons than
progestin-only users (7). Another
advantage of CICs is that there is low incidence of amenorrhoea, after their
prolonged use. Despite all these advantages, this method is not very popular
yet, and is not licensed in many countries. Nevertheless, it can be very useful
in developing countries as it needs less compliance than the oral contraceptive
pill.
Subdermal contraceptives
Research in subdermal implants
started in 1967 which resulted in the introduction of Norplant in 1983 in Finland, before being used in other
countries. It was licensed for 5 years, but has been withdrawn from the UK in
1999. Another discontinued brand was Jadelle,
which was made of two rods each containing 75 mg levonorgestrel. The only
licensed brand in the UK now is Implanon, which is a single subdermal 4 cm rod with 68 mg etonogestrel
dispersed in ethylene vinyl acetate core. It is covered by 0.06 mm
rate-controlling membrane. During the
initial stages, it releases 60 - 70 µg/day, which declines to 25 - 30 µg/day by the end of the third year. It is licensed to be used for 3 years after insertion, and
should be inserted subdermally usually in the upper arm under aseptic
conditions. The same incision used for removing the old rod can be use to
insert the new one. It has the same mode of
action as depoprovera, and almost similar indications as well. There is also increased
risk of irregular uterine bleeding, which makes the commonest cause for
removing the rod. In all, good cycle control was reported by only 28% of women
over a period of 3 years (8). Other statistics showed
30-40% amenorrhoea throughout the 3 years, 30% infrequent bleeding and 10-20%
prolonged bleeding episodes. Its contraceptive and cycle control
efficacy is decreased if
hepatic enzymes inducing drugs such as rifampin, carbamezapine, phenobarbitol
and St. John’s Wort are used at the same time.
Transdermal contraceptives
In
recent years transdermal combined oestrogen and progestogen contraceptive
patches became available. They have the same mode of action and efficacy as the
combined pill, regarding suppression of ovulation, cycle control and cervical
mucous viscosity (10). They have an extra advantage, as the absorbed hormones do not pass
through the liver first. This reduces the production of all oestrogen dependent
hepatic factors, which occurs following the first pass through the liver. A
once-weekly Evra patch (Janssen-Cilag Ltd) can be used for 3 weeks
starting on the first day of menstruation, followed by one patch-free week.
Each patch delivers 33.9 µg ethinyl oestradiol and 203 µg norelgestromin daily
into the systemic circulation. To reduce the risk of ovulation the patch should
be changed every 7 days irrespective whether menstruation has not started or
bleeding has not stopped yet. A new patch may need to be replaced in less than
2% of the cases because of complete detachment. Contraindications for using
combined oral contraceptives are valid for the patch as well. Furthermore, one
follow up study showed that women younger than 20 years of age were less likely
to use the patch properly, compared to the contraceptive pill (11). This method is
more suitable for women in their twenties or thirties who are not very
compliant with the daily routine of the oral contraceptive pill, and are not
keen on other methods.
Intrauterine
devices
1. The mirena system (Bayer) is a levonorgestrel impregnated
intrauterine system which is becoming widely used for contraceptive purposes,
as well as for control of excessive menstrual blood loss. It is made of a
polymer cylinder containing 52 mg of levonorgestrel mounted on a T-shaped
frame. It releases 20 µg of levonorgestrel every day into the uterine cavity,
through a hormone rate limiting membrane covering the device. It acts as a
contraceptive through the same 4 mechanisms described for other progestogens
earlier on in this chapter. It does not inhibit ovulation in the majority of
cases. There is occasionally an initial period of abnormal uterine bleeding
which usually settles within 3 months. Patients should be counselled
accordingly to prevent unnecessary premature removal of the device. Eventually,
the amount of blood loss and bleeding days will be reduced, and 20% of women
may become amenorrhoeic after one year (12). This
beneficial effect has been used to avoid surgery with reasonable results in patients with uterine fibroids. Few
studies showed reduction in blood loss and regression of the uterine size, with
or without diminution in the fibroids mass (13, 14).
This effect was thought to be secondary to inhibition of the endometrial growth
factors. Nevertheless, such devices can be used only when the cavity is not
significantly affected by any fibroid. The system is licensed for 5 years
following its insertion into the uterine cavity. Unlike cupper containing
intrauterine contraceptive devices, it is not licensed for postcoital emergency
contraception.
The mirena system has
no metal component and accordingly different ultrasound characteristic to CuT
devices, but still more echogenic than a Vcu200 device. Figures 42 - 44 show sagittal uterine transvaginal ultrasound views
with a CuT device, mirena system and Vcu200 device correctly sited inside the
cavity respectively. Note the progressively softer echogenic patterns created
by the mirena system and Vcu200 device compared to the sharp echo created by
the CuT.
When available, 3D
ultrasonography can be helpful in showing the exact type of IUCD, especially
with the less commonly used and less echogenic Vcu200 device.
|
|
|
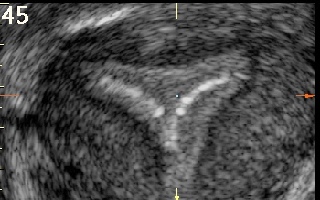
Figure 45
is coronal transvaginal ultrasound scan of a uterus with a normal triangular cavity and Vcu200 contraceptive device in situ.
|
Intravaginal contraceptives
Vaginal rings with
combined oestrogen and progestogen, and progestogen only medication are gaining
popularity as reversible contraceptive means in certain parts of the world. One
example of a combined oestrogen and progestin ring is NuvaRing (Schering), which is available
in the United Kingdom market. It is a flexible one size ring (54 mm) made of ethylene
vinylacetate copolymers and magnesium stearate. It
liberates 15 µg ethinyl oestradiol and 120 µg etonorgestrel daily into the
vagina. It should be inserted into the vagina on the first day of menstruation
by women who did not use hormonal contraception in the preceding cycle. On the
other hand, women who are using any form of combined hormone contraception can
switch to use the ring on any day, the latest being the day following the usual
hormone free interval. It can be used for 3 weeks, followed by seven ring-free
days every month. Personal application is easy by squeezing the ring during its
insertion into the vagina, while squatting, lying down, or with one leg lifted
up on a chair. It does not need to stay in any specific shape or position
within the vagina. Its mode of action is similar to other combined
contraceptives. The ring can be removed for cleaning but replaced within 3
hours to maintain proper contraception. Good cycle control was reported by 98%
of users (15). Nevertheless, 2-5% reported device-related problems including
vaginal discharge, vaginal discomfort and problems during intercourse in the
same study. This last problem can easily be overcome by removing the ring
during intercourse, but replacing it immediately thereafter. The grace period
for changing vaginal rings is longer than for transdermal patches. Accordingly,
one ring can be used continuously for 4 weeks, and changed immediately on the
same day after that with a new one, to avoid onset of menstruation if
desirable. Leaving the same ring in the vagina for more than 4 weeks reduces
its contraceptive efficacy, as advised by the manufacturer’s patient
information sheet. There is also a small risk that NuvaRing can be accidentally
expelled from the vagina during intercourse, with straining during a bowel
movement, and while removing a tampon.
Progestogen-only
vaginal rings are also available in certain
countries, with different durations of action. They act through their
progestational effects on the cervical mucous and endometrium, as for other
progestogen contraceptives. Progering (Andromaco Laboratories, Chile) is a
brand suitable to extend the contraceptive effectiveness of lactation in
breastfeeding women. It was initially tested for 3 months (16), but a more recent study showed that it is
effective for 4 months without affecting breast-feeding or the rate of infant
growth. It also prolonged the period of lacational amenorrhoea (17). The ring can be removed for comfort during sexual
intercourse, but additional contraception will be necessary for one week if it
has been removed for more than 3 hours. Bleeding disturbances are common, as
for other progestogen only contraceptives.
Efficacy of hormonal contraceptive
The statistics used to
compare the efficacy of different contraceptive methods is usually documented
as failure per hundred women years. This estimate of efficacy refers to the
first year of use, though the longer a woman uses a contraceptive method, it is
less likely for that method to fail. It is important to understand that failure
rate depends on many factors which can reduce the efficacy of the contraceptive
method. Parts of these factors are related to the method itself, but a major
part is related to the way it has been used. This is reflected by failure in
relation to the typical use and perfect use of the specific contraceptive.
Perfect use is a measure of efficacy when the method has been used perfectly
according to the manufacturer’s guidelines, without fail. Failure in such cases
reflects the inherent capabilities of the method itself. On the other hand,
typical use failure rate reflects the probability of pregnancy during the first
year, allowing for non-compliance and incorrect use of the method. This is the
statistics usually quoted in the literature, which is obviously affected by
many confounders. It is understable why long acting contraceptive methods which
rely less on patients’ compliance offer similar typical and perfect use failure
rates. The perfect use failure rates of depoprovera and Norplant are 0.03% and 0.05%
respectively, with the typical use failure rates being almost identical. This
is in contrast to the other methods which depend on the patients’ compliance.
The combined oral contraceptive pills perfect use failure rate is approximately
0.1-0.5%, but the typical failure rate is as high as 5%
(18).
Non
contraceptive uses of hormonal contraceptives
Family planning remains to be the most common
indication for using hormonal contraceptives. Nonetheless, patients who need
such protection may have other gynaecological or chronic medical problems,
which make proper selection of a specific brand with a specific route of
administration more important. These issues will be discussed in the following
sections of this chapter.
2. Cycle control in
oligomenorrhoea or amenorrhoea is an indication to prevent
endometrial hyperplasia and hypo-oestrogenic state. This should be done after
making a proper diagnosis of the initial cause of anovulation. This is
especially so for thyroid dysfunction and hyperprolactinaemia, as treatment of
these conditions is most likely to correct the menstrual problem.
Oligomenorrhoeic and amenorrhoeic women with polycystic ovary syndrome are at
risk of developing endometrial hyperplasia. This increases the risk of
endometrial carcinoma. Using an oral contraceptive pill
to induce regular monthly withdrawal bleeding will reduce this risk.
Alternatively, a mirena system can be used instead. On the other hand, young
women with hypo or hyper gonadotrophic hypogonadism are at risk of developing
hypoestrogenic side effects including osteoporosis. One option to deal with this
problem is to use a low dose oral contraceptive brand.
3. Treatment of
abnormal uterine bleeding especially excessive blood loss is also an indication
for using an oral contraceptive pill, or the mirena system. This is especially so in
patients with dysfunctional uterine, when no specific treatable endocrine or
organic uterine causes can be detected. This subject has been discussed in
detail before in this chapter under the subtitle ‘Intrauterine contraceptive
devices’.
4. Treatment of
hyperandrogenisation is an important indication for using non androgenic oral
contraceptive pills. This is affected by inhibiting ovulation, reducing ovarian
androgens production, and increasing the level of SHBG which reduces the level
of free testosterone. It is also important to exclude other specific treatable
conditions which may increase the level of serum androgens, including ovarian
and adrenal tumours. Adult onset adrenal hyperplasia should also be excluded,
as treatment usually entails suppression of the adrenal androgens as a
priority. The highest level of SHBG is reported after using a combined pill
with cyproterone acetate as the progestogen fraction (Dianette, Bayer
plc). The role of
non androgenic progestogens has already been discussed in this chapter. Pills
with low progestogen / oestrogen activity are preferable in the treatment of
patient with acne, but may not give the best cycle control, and breakthrough
bleeding is more likely. The direct antiandrogenic effect of drospirenone in reducing 5a reductase
activity is well utilised in the combined pill Yasmin (Bayer plc).
5. Using an oral
contraceptive pill to inhibit ovulation has been shown to reduce the risk of
developing functional ovarian cysts while under treatment (19). This effect has been well documented
for high dose older contraceptives with 50 µg ethinyl oestradiol, but not for
the lower dosage group. Once a cyst was formed such treatment achieved similar
results to expectant management (20, 21). A
significant similar effect has also been found with other benign ovarian tumours including serous and mucinous adenomas, teratomas
and endometriomas (22). The reduction in risk
was related to the duration of use. Similar to functional cysts, oral
contraceptives are not useful for the treatment of these benign ovarian cysts
once they already developed.
6. Oral
contraceptive pills are also used in cycle preparation during assisted
reproduction treatment cycles. This is done usually in cases with irregular
menstruation for good planning of the treatment cycles. One protocol involves
the use of an oral contraceptive pill after a progestogen withdrawal bleeding episode. This is followed by daily injections
of a gonadotrophin releasing hormone (GnRH) agonist, from day 17 of the cycle
to affect down regulation of the pituitary gland. Nasal sprays can be used
instead. Controlled hyperstimulation is started within a day or two after the
withdrawal bleeding has started, usually within a week after stopping the pill.
There will be less risk of developing an ovarian cyst following the GnRH
agonist during such a protocol. Accordingly, a similar protocol can be
prescribed for patients who developed ovarian cysts following GnRH agonist use
during previous assisted reproduction treatment cycles.
7. The use of
oestrogens and combined oral contraceptive pills in the management of young
women with absent or delayed pubertal development has been discussed in Chapter
2. Unopposed oestrogen is usually a priority for some time till normal height
has been attained, before converting to an oral contraceptive pill.
8. Low dose combined
contraceptive pills can also be used as hormone replacement therapy in cases of premature ovarian failure as
discussed in Chapter 10. These patients usually need a higher oestrogen dose
than women who went through natural menopause. Furthermore, having regular
withdrawal bleeding has a good psychological effect on these patients. Neither
the combined contraceptive pills nor any designated hormone replacement therapy
are effective as contraceptives in these cases. Pregnancies have been reported
in young women with premature ovarian failure while on the pill. This
information should be conveyed to all patients in such a situation who are
adamantly keen not to get pregnant. Women with anorexia nervosa are at special risk of severe hypoestrogenism.
Using a low dose combined contraceptive pill will provide the missing
oestrogen, while the patient is having the necessary psychological treatment.
This is also valid for professional women athletes, who are at risk of the
triad of amenorrhoea, anorexia and osteoporosis.
9. Medical treatment of endometriosis also involves using different types of
hormonal contraceptives. Cyclic or continuous use of oral contraceptive pills
are two options. Recently, the extended use of an oral contraceptive pill for
84 days has been introduced. Depoprovera and the mirena system are also useful in this respect. The dominant
progestational effect of all these drugs is the main factor in controlling
endometriosis growth and symptoms. This does not give permanent cure, and
symptoms and signs usually recur after medication is suspended. Furthermore,
such medication is not suitable for women who are keen to conceive, which is a
real limitation for this therapy.
10. The role of
hormonal contraceptives in the management of premenstrual dysphoric disorders
has been discussed in Chapter 10. Suffice to say that mixed results have been
reported about the effect of the oral contraceptive pill, and the risk of
worsening symptoms during the pill free period. This resulted in the release of
brands with 24 and 26 pills to reduce the pill free period. Using an extended
brand for 84 days, like Seasonale (Duramed Pharmaceutical, Inc), has also been
shown to improve premenstrual dysphoric disorders. It contains 84 active pills,
each containing 30 µg ethinyl oestradiol and 0.15 mg levonorgestrel, with 7
inert tables. A pill which gained notoriety in this respect is Yasmin (Bayer
plc), as the
progestogen fraction has been replaced with drospirenone which is a
spironolactone derivative. Each pill contains 30 µg ethinyl
oestradiol and 3 mg drospirenone, and used for 21 days with one pill-free week.
Another pill with 20 µg ethinyl oestradiol and 3 mg drospirenone with 24 active
pills is marketed in America, and used especially for premenstrual dysphoric
disorders. In very severe cases, the mirena system can be used, together with continuous
transdermal oestrogen.
11. The mirena system has a wide range of non contraceptive benefits
which have been alluded to before in this chapter. One benefit not mentioned
before, is its role in protecting the endometrium against the chronic
hyperplastic effect of tamoxifen during treatment of breast cancer. Other
benefits include control of endometriosis induced pelvic pain, and for endometrial
protection in postmenopausal women on continuous oestrogen replacement therapy. An extra important benefit of the mirena system is
its effect in reducing the symptoms and size of endometriotic lesions in the
rectovaginal septum (23). This will obviate the
need for the difficult surgery necessary to deal with this pathology in most
cases. Furthermore, suggestions have been put forward to use it immediately
after endometriosis surgery to reduce the need for further operations in the
future (24). Levonorgestrel has been found in
the peritoneal fluid in significant amounts, approximately two thirds of the
serum level, in women who showed improvement in their symptoms after six months
of using a mirena (25). Similarly, the level of
the serum marker CA-125 showed equivalent decline after long term use of the
device as for GnRH-a, when used for the treatment of endometriosis (26). All these effects were related to the high levels
of peritoneal levonorgestrel causing increased programmed cell death
(apoptosis), atrophy of the ectopic endometrial glands, and decidual
transformation of the stroma. It has also been reported to have
anti-inflammatory and immunomodulatory effects (27).
Furthermore, levonorgestrel has been shown to decrease and then block DNA
synthesis and mitotic activity (28).
Other coincidental benefits of hormonal
contraceptives
There are
many coincidental health benefits related to the use of the oral contraceptive
pills including:
• An epidemiological study reported by Schlesselman in 1991 (29) showed a
duration-related protective effect of combined oral contraceptive pills against
endometrial cancer. The risk before
the age of 60 years was reduced by about 38% with two years of use. Longer use
for 4, 8, and 12 years, conferred 51%, 64%, and 70% reduction in endometrial
cancer risk respectively. Such protective effect lasted for ≥15 years after
stopping medication.
• Many reports documented a protective effect of oral contraceptives
against ovarian cancer in general. This risk also decreased with increased
duration of use. The adjusted odd ratio for ovarian cancer with any past use of
oral contraceptives was 0.5 (95 CI 0.3 – 0.8). A 60% risk reduction was noted
after 6 years of use or more (30). Such
protective effect was noted even in carriers of BRCA1 (odd ratio, 0.5; CI 0.3 –
0.9), and BRCA2 mutations (odd ratio , 0.4; CI 0.2 – 1.1)
• Reduced risk of functional ovarian cysts is another benefit related to
suppression of ovulation. Risk reduction was also related to the duration the
oral contraceptives use. Such benefit persisted for at least 15 years after
stopping using the pill. The protection rate in comparison to non-users has
been estimated as 30% for women who used the pill for 4 years or less. This
figure increased to 50% and 80% for 5 – 11 years, and >12 years of the pill
use respectively (31, 32).
• Reduced risk of colorectal cancer has been documented for patients
who used oral contraceptives with 50 µg ethinyl oestradiol, with a relative
risk of 0.6 after 96 months of use. The effect of low dose brands has not been
verified.
• Decreased incidence of benign breast diseases including fibrocystic
changes and fibroadenomas has been attributed to the use of oral combined
contraceptives. This effect was seen even after only one or two years of use (33), and lasted for one year after stopping the pill (34). Conversely, the ESHRE Capri Workshop Group in 2005 (35) thought there
were significant problems with bias, study design and interpretation of results
related to this topic. Furthermore, they thought there was no evidence of
biological plausibility. In short, they did not endorse the idea of a
beneficial effect on the breasts.
• The subject relating bone mass density to hormonal contraception has
generated a lot of contradictory results. The osteoporotic effect of
depoprovera has been discussed already. Oral
progestogens only pills do not inhibit ovarian function, and do not have
similar effect on bone density. The difficulty with studies which addressed
bone mass density has been confounded by different factors which affect bone.
Examples of such factors included hereditary factors, age, body mass index,
smoking, level of physical exercise and diet. On the other hand, there is some
evidence of a little favourable effect of the combined oral contraceptives on
bone mineral density and fracture risk in women of reproductive age as
concluded by the ESHRE Capri Workshop Group (35).
This is despite of the fact that oestrogen blood concentrations over the whole
month are lower in women using the combined oral contraceptive pill than during
natural ovulatory cycles. They did not endorse any benefit on bone mass density
in patients with anorexia nervosa.
Use of
hormonal contraceptives by women with known medical problems
Young women with different non gynaecological medical
problems may need contraceptive advice to prevent unplanned or unwanted
pregnancies. This occasionally creates different difficulties, because of the
direct effect of the method used on the medical condition, or its interaction
with the different drugs necessary to control her basic medical problem. The
most likely conditions to be encountered in this age group include obesity,
high blood pressure, diabetes mellitus, migraine, hypothyroidism, pituitary
adenomas and thrombophilias. Patients with certain risk factors may also
request contraceptive advice. This group includes women with previous history
of deep vein thrombosis, and those with personal or family history of breast
cancer
Obesity
It is not uncommon for obese patients to seek
contraceptive advice. They may even have pre perceived ideas against using
barrier methods for different reasons. A more urgent situation can arise when
an obese patient presents with irregular or excessive menstrual blood loss,
which needs immediate hormonal treatment and cycle control in the long term.
Obesity is a known risk factor for venous thrombosis, pulmonary embolism,
insulin resistance, and diabetes mellitus. There is
increased risk of thromboembolism in women with BMI >35 kg/m2.
The Royal College of Obstetricians and Gynaecologists considered a BMI of 40
kg/m2 or more as a contraindication to use oral contraceptives. In
fact the risks of using oral contraceptives generally outweigh their benefits
for women with BMI of 35 to 39 kg/m2. This is coupled with a
negative correlation between BMI and the contraception effectiveness of
transdermal patches and the vaginal ring, leading to higher failure rates. This
may be due to altered steroid metabolism, and / or dilution of the steroid dose
in a larger blood volume. The evidence for an association between oral
contraceptives failure and obesity has been conflicting. This subject has been
reviewed by Brunner et al in 2006 (36). They
confirmed a negative association, but the results were largely attenuated after
adjustments for age, ethnicity and parity. Taking all the risks involved into
consideration, it seems that the preferred option for obese women is a mirena
system. The revised
Depoprovera Product Monograph in 2006 (3)
reported variable results related to weight gain after using the product. The
majority of studies reviewed showed weight gain of 5.4 lbs (2.5 kg) at the end
of one year. Other studies reported weight gain of 8 lb (3.5 kg) by the end of
the second year, but 20-40% of depoprovera users actually lost weight during treatment.
High
blood pressure
High blood pressure is another possibility in
patients seeking contraceptive advice. It is a risk factor for cardiovascular
events and stroke, even in young women, who are normally expected to have a low
risk (37). The estimated annual
incidence of myocardial infarction and strokes has been reported as 1.7 and
34.1 cases per 1 million respectively, for normotensive women aged 30–34 years.
These rates increased to 10.2 for myocardial infarction and 185.3 for stroke
among hypertensive women of the same age group (38). Women using combined oral contraceptives are at increased
risk of developing high blood pressure. Certain factors are known to increase
this risk, including a strong family history of high blood pressure especially in female relatives, and history of high blood
pressure during a previous pregnancy. Other risk
factors include racial origin, age, obesity, smoking and alcohol abuse. Duration of the oral contraceptives use is also
important. There were higher age-adjusted blood pressure levels after 8 years
of use than in women who used the pill for shorter periods of time as reported by
Lubianca et al in 2003 (39). The same authors concluded that hypertensive women
on oral contraceptives had significant elevation of diastolic blood pressure
and poor blood pressure control, independent of age, weight and
antihypertensive drug treatment. In a subsequent publication Lubianca et al in
2005 (40) reported reductions of 15.1+/-2.6 mm Hg and 10.4+/-1.8 in systolic and
diastolic blood pressure respectively after stopping oral contraceptives use.
They recommended stopping such medication as an effective antihypertensive
intervention in such a clinical setting. This finding was agreeable with another
study reported by Atthobari et al in 2007 (41) who
showed worsening of high blood pressure with hormonal contraceptives and its
improvement after discontinuation of medication. It also showed some
deterioration in renal function during usage of hormonal contraceptives. Urine albumin excretion increased
by 14.2% in starters (P = 0.074), and fell by 10.6% after stopping
medication (P = 0.021). On the other hand, the glomerular filteration
rate fell by 6.3% in starters (P < 0.001), and did not recover after
stopping the contraceptive. The same authors reviewed previous studies for the
correlation between hormonal contraception and renal function, in relation to
these findings. Two previous studies proposed that contraceptives use may be
associated with an increased risk of microalbuminuria, independent of the blood
pressure effect. On one hand, higher levels of albuminuria are considered as an
early marker of vascular endothelial damage, but there is no significant
evidence relating renal disease to the use of hormonal contraception. Both
pathologies are related to an increased risk of progressive renal failure and
excess cardiovascular morbidity and mortality (41).
This
section can be concluded that the combined oral contraceptive pill is better
avoided by hypertensive patients, and by women liable to develop high blood
pressure. Progestogen only contraceptives can be used instead. Nonetheless,
controlled mild high blood pressure as an isolated problem is not an absolute
contraindication against using a low dose oral contraceptive pill. Readjustment
of the antihypertensive treatment dose may be necessary, due to stimulation of
the renin angiotensin system and aldosterone production by oestrogens. At
the same time, regular medical supervision will be needed, if the patient opted
to continue with the same medication, rather than using an alternative method.
Conversely, uncontrolled or complicated hypertension is an absolute
contraindication to use the combined oral contraceptive pill. Other risk
factors like obesity and smoking should be taken into consideration when making
the decision.
Thrombophilia
and history of thromboembolism
Thrombophilia is a term used to indicate increased
tendency to intravascular coagulation. It may follow hereditary or acquired
causes. Congenital factors include factor V Leiden mutation, prothrombin, protein C and S, and
antithrombin III deficiencies. High homocysteine and sickle cell disease are
other factors to consider. Thombophilia can also be acquired due to
antiphospholipid antibodies. Factor V Leiden forms the most common inherited
thombophilia, and 3 – 8% of
Caucasians carry a copy of the mutation in each cell. Furthermore, 1 in 5000
individuals carries 2 copies of the mutation. The risk of thromboembolism for
Factor V Leiden is >10 times more (80%) in this group than for the
heterozygous state (7%). It is less common in other ethnic groups. It is
important to note that not all thrombophilias carry the same risk of venous
thromboembolism. The lowest risk is attributed to a single mutation such as
Factor V Leiden, and the highest to antithrombin deficiency (42). Using the combined oral contraceptive pills is
associated with a small increase in thrombosis and pulmonary embolism risks. In
numerical terms, about 20 – 30 women in every 100,000 pill users may develop
deep vein thrombosis or pulmonary embolism. Previous history of either problem
or a personal history of thrombophilia will increase the risk. Despite the
controversy regarding the increased risk with third compared to second
generation oral contraceptives, epidemiological data confirmed this increased
tendency for venous, but not arterial thrombosis (43).
Furthermore, the increased risk of venous thromboembolism is more pronounced
during the first year of use. Other contributing factors included obesity,
cigarette smoking, immobility, fractures, surgery and cancerous conditions
especially of the pancreas. There is an exponential increased risk when genetic
factors are combined with these conditions. Accordingly, great care should be
taken to identify patients with such genetic risk factors, but screening all
women is not indicated before prescribing oral or other hormonal
contraceptives. Additionally, oestrogen containing pills should be avoided in
these cases when contraception is needed. Though progestogen contraceptives,
whether oral or injectable, do not increase the thrombosis tendency in the
general female population, their role in individual women with thrombophilia
has not been investigated. Accordingly, there is an opinion that a
copper IUCD should be the first-line contraceptive method for women with a
history of deep venous thrombosis, pulmonary embolism, or coronary events (44).
Diabetes
mellitus
Classical teaching has always associated
combined oral contraceptives, and in some cases even the progestogen only
brands, with changes in carbohydrate metabolism. Such changes included
decreased glucose tolerance and increased insulin resistance, which are risk factors
for type II diabetes mellitus and cardiovascular disease. This issue has been
addressed by a meta-analysis published by Lopez et al in 2009 (45). They concluded
that the current evidence showed a limited effect for hormonal contraceptives
on carbohydrate metabolism in women without diabetes. Furthermore, they
criticised many of the published articles because of the small numbers of women
involved, lack of comparison between different types of contraceptives, and
lack of information regarding the effects among overweight women. In the same
year, Cagnacci
et al (46) found that vaginal contraceptive
rings were less likely to change insulin resistance than combined oral
contraceptives. They concluded that a vaginal ring may be a better choice for
long-term contraception in women at risk for developing diabetes mellitus or
the metabolic syndrome. In a study which addressed the effect of depoprovera injections, Xiang et al found increased risk
of diabetes which they explained by three factors (47):
- The drug was used by women with increased
baseline diabetic risk;
- There was associated weight gain during
medication;
- The drug was used in cases with high baseline
triglycerides and/or during breast-feeding.
All this information
indicates that proper selection of the suitable contraceptive should always
take into consideration the inherent characteristics of the drug to be used,
and the risk factors shown by each individual woman.
Contraception is an
important issue for diabetic women, as unplanned pregnancy can induce major
maternal and perinatal complications. There was little evidence that changes in
blood sugar control induced by combined oral contraceptives had any clinical
consequence as reported by Shawe and Lawrenson 2003 (48). Furthermore, low
dose combined contraceptive pills have minimal effect on the lipid profile
which may even by beneficial. Conversely, there is some concern regarding the
effect of oral and injectable progestogens on HDL and LDL cholesterol levels. Diabetes mellitus
is a risk factor for thromboembolism and cardiovascular diseases, but well
controlled diabetes is not an absolute contraindication for using the oral
contraceptive pill. The same authors (48) stated
that short term studies of young women with uncomplicated
diabetes who were taking low-dose combined oral contraceptives have been
reassuring. On the other hand, patients with complicated diabetes and
macrovascular or microvascular changes should be prescribed nonhormonal
contraceptive methods. The same is valid for cases with uncontrolled diabetes, or
in the presence of other risk factors like obesity, hypertension or smoking. In
a different approach Nikolov et al in 2005 (49) made a clear
distinction between types I and II diabetics in relation to contraceptive
advice. They recommended the use of low dose
combined contraceptive pills in women with uncomplicated type I diabetes of
less than 15 years duration. A change in the insulin dose and control of body
weight will be needed to maintain good glycaemic control. Conversely, they
advised against using combined oral contraceptives in women with type II
diabetes, because they may provoke clinical changes and
worsen the progress of the disease itself. This last point is very much valid
for prediabetics and diabetics controlled only by diet. Further deterioration
of the glycaemic condition, which may follow using combined contraceptives,
adds a further burden on the patient to take hypoglycaemic drugs, which also
adds cost and the need for more strict compliance.
Complications
and risks of hormonal contraceptives
This section will be dealt with in
bullets form to focus the attention of the reader, as most points have already
been addressed before in this chapter. Few of the complications are
gynaecological in nature, but systemic and organ specific complications or
risks will also be addressed.
- Dysfunctional
uterine bleeding may follow inappropriate or prolonged use of oral
contraceptives. This is also valid for progestogen only contraceptives
whether oral, injectable or implants. It is not unusual for dysfunctional
uterine bleeding following depoprovera to be unresponsive to treatment with
oestrogen or tranexamic acid. Use of further progestogens either as norethisterone
or medroxyprogesterone acetate may even increase the bleeding problem.
- Long term
amenorrhoea and delayed return of fertility potential can follow
depoprovera injections. Though 9 months after the
last dose was a figure usually quoted for return of fertility, many women
do not menstruate for much longer periods of time.
- There is
increased risk of thromboembolism in obese patients and smokers and in
women >35 years of age, especially with the combined contraceptives as
discussed before.
- Osteoporosis
with long term injectable progestogens is a risk factor. It can be more
significant in very lean young teenage girls as the maximum bone density is usually attained by the age of 20
years.
· Unfavourable
changes in cervical cytology have been seen in women using combined oral
contraceptive pills. Furthermore, prolonged use of these pills has been
associated with a small increased risk of cervical cancer. Among current users
of the pill, the relative risk (RR) after 5 years of use was 1.9 (95% CI,
1.69-2.13) compared to those who never used the pill. There was gradual decline
in the risk, which returned to normal 10 years after stopping the pill. This
pattern was seen in invasive and in situ cancers and for women who tested
positive for the high risk human papilloma virus, as reported by the
International Collaboration of Epidemiological Studies of Cervical Cancer in
2007 (50). The
same group estimated an increased cumulative incidence of cervical cancer from
3.8 to 4.5 per 1000 women by the age of 50 years in women who used the pill for
10 years between the ages of 20 and 30 years, in developed countries. The
corresponding increase was from 7.3 to 8.3 per 1000 for developing countries.
The reason for this increased risk of cervical cancer was not well elucidated,
but was thought to be an association rather than a cause and effect
relationship. Women using the pill are more likely to be sexually active and
may not use barrier contraceptive means regularly. This can put them at
increased risk of contacting human papilloma virus, which is the likely cause
of cervical cancer. Accordingly, it is important that all women on combined
oral contraceptive pills should have regular cervical smear screening,
especially if human papilloma virus infection was detected (51). A note should be documented here about the effect
of depoprovera. An overall non
significant relative risk of 1.11 (95% CI, 0.96 – 1.29) has been reported for
invasive sqaumous cell cervical carcinoma in women who ever used the drug. This
was not affected by the duration of use, or the times since the initial or most
recent injection (2).
· The issue
relating breast cancer risk to the oral contraceptive pill is more complicated
than the pill’s relationship to cervical cancer. It is a subject which had
attracted, and still attracts the attention of the media and public. Many
scares have hit the public in waves since the 1970s, which lead to thousands if
not millions of unplanned or unwanted pregnancies, and equally distressing
terminations of pregnancies. The relationship has been confounded by the
multifactorial nature of breast cancer, which is affected by many variables. The odd ratio is 200 for those
with the BRAC gene mutation. A figure of 3 has been reported for women with
familial history of breast cancer (52).
Furthermore, many life style and other related variables have been known to
increase the risk. The list includes lack of exercise, excessive alcohol
intake, cigarette smoking, postmenopausal obesity, early menarche, late
menopause, first full-term pregnancy after the age of 35 years, and reduced
breast feeding (53). Each of these factors increased the risk
of breast cancer more than the risks reported for combined oral
contraceptives (53). As an example of such
confounders, women who had their menarche before the age of 12 years had 30%
higher risk of developing breast cancer than those who started menstruating by
the age of 15 years, as reported by ESHRE Working Capri Group (35). A meta-analysis
published by Kahlenborn et al in 2006 (54) examined 34 studies that met stringent inclusion
criteria since 1980, for an association between using oral contraceptives and
premenopausal breast cancer in general. A small increased risk with an odd
ratio (OR) of 1.19 was found (95% CI, 1.09-1.29). Both parous (OR, 1.29; 95%
CI, 1.20-1.40) and nulliparous (OR, 1.24; 95% CI, 0.92-1.67) women were
affected. Prolonged use did not change the OR risk for nulliparous women. The
risk was stronger when the pill was used before the first full term pregnancy.
Furthermore, the maximum risk was seen when the pill was used 4 years before
the first full term pregnancy, with an OR of 1.52 (95% CI; 1.26-1.82). Despite
all these figures, the risk of breast cancer remains to be small, and the
benefits of using the oral contraceptive pill outweigh their risks in most
women. To put this statement into mathematical perspective, there will be 0.5
additional breast cancers per 100,000 women 16-19 years of age, during the time
of use and 10 years follow up. The corresponding figures for women in the age
groups 20-24 years and 25-30 years are 1.5 and 4.7 additional cancers per
100,000 respectively as reported by Reid in 2007 (53).
Emergency
contraception
Emergency or postcoital contraception
should be used to prevent unwanted pregnancy after unprotected intercourse. It
is an occasional method and should not be used as a regular means of
contraception. Copper intrauterine contraceptive devices are the most efficient
means if inserted within 5 days of unprotected intercourse, with a failure rate
<1.0% (55). They act mainly by preventing
fertilisation through the toxic effect of copper on sperm, but impede
implantation at the same time. TT380 Slimline (Durbin PLC, South Harrow, Middlesex, UK) and
T-Safe 380A (Williams Medical Supplies Ltd, Rhymney,
Gwent, UK) are the most effective, and have the lowest failure rate. They are
the preferred method for women using liver enzymes inducing drugs, such as
barbiturates, carbamazine, rifampicin and phenytoin. Nulliparous women could be
offered the Mini TT380 Slimline, which has a small plastic frame.
The two main hormonal means are Levonorgestrel
1500 µg tablets and ulipristal acetate which is a synthetic second generation
selective progesterone receptor modulator. The levonorgestrel contraceptive
pill (Levonelle 1500, Bayer Schering)
is licensed for use within 72 hours of intercourse. It prevents pregnancies in
95%, 85% and 58% of the cases if used within 24, 24 – 48 and 48 – 72 hours
after the first intercourse respectively (56).
It interferes with follicular development and impairs ovulation, with little
evidence regarding inhibition of implantation. Another tablet should be taken
if vomiting occurs within 3 hours after taking the pill. It is also recommended
that a woman on liver inducing enzymes should take 2 tablets soon after
unprotected intercourse, if she is not agreeable to use a copper IUCD. Ulipristal
acetate (30 mg tablet) could be used up to 120 hours
after intercourse, and is more effective than levonorgestrel for inhibiting
ovulation (57, 58).
It also affects implantation, and accordingly could be used for emergency
contraception after ovulation but before the expected time of implantation. No
teratogenic effects have been attributed to either drug, and termination of
pregnancy is not indicated on this context in cases of failed contraception. Both
drugs could lead to menstrual irregularities and delay of menstruation, which
may cause patients some concern.
An alternative method of emergency hormonal
contraception is to take 4 combined oral contraceptive pills immediately after
intercourse and repeat the dose 12 hours later. Microgynon 30 (Bayer Schering)
has been recommended for this purpose (59). This
may lead to nausea and vomiting, and an anti-emetic drug may be needed in these
cases. This method is indicated if the single-tablet protocols or copper IUCD
are not available or unacceptable.
Perimenopausal
contraception
Previous studies showed lower tendency by
women over the age of 40 years to use contraception, with 40% opting for
sterilization in the United Kingdom (60). This
pattern may change because of the introduction of newer methods of
contraception. It is important to emphasise that no method of contraception is
contraindicated on the basis of age only, in the absence of risk factors or
medical problems. Perimenopausal women are more likely to develop high blood
pressure, diabetes mellitus, obesity and dysfunctional uterine bleeding which
make selection of an appropriate contraceptive method rather difficult. At the
same time, pregnancy at this age carries great risks for the mother and fetus,
and appropriate contraception should be guaranteed to prevent unplanned
pregnancies. The following points should be taken into consideration when
offering contraceptive advice to women in this age group:
·
It is most important to take a woman’s preference into
consideration and to discuss with her the merits and drawbacks of her chosen
method of contraception to improve compliance. Certain taboos may be attached
to the use of certain methods which make them less acceptable in different
areas and cultures.
·
Women above the age of 40 years can use combined hormonal
contraceptives up to the age of 50 years, unless there are coexisting risk
factors. They should be switched to another form by that age. The last
statement is also valid for women using injectable progestogen only
contraceptives, but inherent predisposition to osteoporosis should be taken
into consideration even in younger women.
·
Smoking is a significant cardiovascular risk factor even
at the age of 35 years and in younger obese women. This risk falls
significantly one year after stopping smoking and almost disappears 3-4 years
later.
·
There is 50% increased risk of thromboembolic attacks
among cigarette smokers independent of oral contraceptives use or age (61).
·
Women with migraine, history of stroke or cardiovascular
disease should not be prescribed combined hormonal contraceptives.
· The progestogen only pill and mirena system are good
options, but there is a higher risk of dysfunctional uterine bleeding with the
former method, which made it unsuitable for many perimenopausal women (62). They are not associated with increased risk of
thromboembolism (61, 63). Both methods can be
used by patients with previous history of ischaemic heart disease and stroke,
but the progestogen only pill should not be used during an active thrombotic
episode. The risks involved by using injectable progestogen only contraceptives
outweigh their benefits in these conditions. The role of copper IUCD in such
cases has already been mentioned before (44).
· Monophasic contraceptive pills with £30 µg ethinyl
oestradiol and low dose northisterone or levonorgestrel are good first line
options for women with no risk factors who opted to use a combined pill. The
risk of venous thrombosis decreases with lower oestrogen dose and duration of
use.
· The non-contraceptive health benefits of low dose
contraceptive pills alluded to before, justify their use in healthy
perimenopausal women (63). Kaunitz viewed their
use in this age group as a general strategy not only to provide effective
contraception, but also to improve perimenopausal symptoms and to enhance
quality of life (64).
· Oral contraceptive pills with desogestrel, gestodene or
drospirenone have significantly higher risk of venous thrombosis than other
brands containing levonorgestrel, as confirmed by Lidegaard et al in 2009 (65).
· In general, the risk of cerebral thromboembolic attacks is
reduced by >30% when using low dose pills compared to 50 µg preparations (61). This is equivalent to one additional death per
one million low dose pill users per year in comparison to IUCD users (66). On the other hand the General Practice Research
Study (67), and the WHO Collaborative Studies of
Cardiovascular Disease and Steroid Hormone Contraception (68, 69) showed no
increase in mortality rate from stroke or venous thrombosis in low dose pill
users.
· A copper IUCD is an alternative non-hormonal method of
contraception, but may exacerbate menstrual problems in this age group (70). The T380 device offered contraceptive
effectiveness equivalent to surgical sterilization (71).
Accordingly, such a device may be offered to women free of any pre-existing
menstrual problems, otherwise the mirena system should be used if this mode of
contraception is preferred (72).
· Combined HRT does not provide effective contraception and
women on such medication should use effective contraception till the age of 55
years. This can be provided by the progestogen only pills or a mirena system.
Two FSH blood level estimations will be needed 6 weeks after stopping HRT for
confirmation of the menopause, before contraception can be stopped (73).
Summary
The subject of hormonal contraception has
been a prime issue in the public domain many times over the last 30 years, but
mostly for the wrong reasons. Women who seek contraceptive advice are mostly
young and healthy, and can use hormonal contraceptives safely in the majority
of cases. This is a different scenario to other patients who have known risk factors,
or are currently under treatment for one or another chronic illness. An
unwanted pregnancy can cause more damage than the small risks of inducing
cardiovascular problems or breast cancer, which are not very common in this
young age group. Furthermore, the thromboembolic risks of pregnancy exceed
those related to the use of the combined oral contraceptive pill. The medical
and psychological risks involved with termination of pregnancy should be taken
into consideration as well. Many women can not even consider the option of
having a termination, because of ethical or religious reasons, and get stuck
with an unwanted pregnancy. Accordingly, the most appropriate contraceptive
should be selected for each individual woman taking into account her own preferences,
any available risk factors, the issues of expenses, compliance, and ease to see
a medical or nursing professional when necessary. The other non contraceptive
benefits mentioned before in this chapter should be added to the whole package
when discussing the issues related to contraception with patients.
References
1. Sangi-Haghpeykar H, Poindexter AN III, Bateman L, Reid ED.
Experiences of injectable contraceptive users in an urban setting. Obstet
Gynecol. 1996; 88: 227 - 233.
2. Product
Monograph. Depoprovera. Pfizer Canada Inc 2006.
3. Pettiti DB, Piaggio G, Mehta
S, Cavioto MC, Meirik O. Steroid hormone contraception and bone mineral
density: a cross-sectional study in an international population: the WHO Study
of Hormonal Contraception and Bone Health. Obstet Gynecol. 2000; 95: 736 - 744.
4. Westhoff C, Britton JA, Gammon
MD, Wright T and Kelsey JL (2000) Oral contraceptives and benign ovarian
tumours. Am J Epidemiol 2000; 152: 242 – 246.
5. Long-acting
hormonal contraceptives. In: FIGO Manual of Human Reproduction, Vol 2. Fathalla
MF, Rosenfield A and Indriso C (ed). The Parthenon Publishing Group. Casterton
Hall, 1989; 67 – 83.
6. Bahamonds
L, Lavin P, Ojeda G, Petta C, Diaz J, Maradiegue E, Monteiro I. Return of
fertility after discontinuation of the once a month injectable contraceptive,
Cyclofem. Contraception 1997; 55: 307 - 310.
7. Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C. Combination
injectable contraceptives for contraception. Cochrane Database of Systematic Reviews 2008, Issue 4. Art No:
CD004568. DOI: 10.1002/14651858. CD004568.pub3.
8. Rai
K, Gupta S, Cotter S. Experience with Implanon in a northeast London family
planning clinic. Eur J Contracept Reprod Health Care 2004; 9: 39–46.
9.
Creasy
GW, Abrams LS, Fisher AC. Transdermal Contraception. Semin Reprod Med 2001;
19(4): 373 - 380.
10. Smallwood GH, Meador ML, Lenihan JP, Shangold
GA, Fisher AC, Creasy GW; Ortho Evra/Evra 002 Study Group Efficacy and safety
of a transdermal contraceptive system. Obstet Gynecol 2001; 98: 799 - 805.
11.Archer DF, Bigrigg, Smallwood GH.
Assessment of compliance with a contraceptive patch (Ortho Evra TM/Evra TM)
among North American women. Fertil Steril 2002; 77: S27 - S31
12. Lahteenmaki P, Rauramo I, Backman T.
The levonorgestrel intrauterine system in contraception. Steroids 2000; 65: 693
- 697.
13. Fong YF and Singh K. Effect of levonorgestrel-releasing intrauterine
system on uterine myomas in a renal transplant patient. Contraception 1999; 60
(1): 51 - 53.
14.Magalhaes J, Aldrighi JM and de Lima GR.
Uterine volume and menstrual patterns in users of levonorgestrel-releasing
intrauterine system with idiopathic menorrhagia or menorrhagia due to
leiomyomas. Contraception 2007; 75 (3): 193 - 198.
15. Roumen FJME, Apter D, Mulders TMT,
Dieben TOM. Efficacy, tolerability and acceptability of a novel contraceptive
vaginal ring releasing etonogestrel and ethinyl oestradiol. Human Reprod 2001;
16:469 - 475.
16. Massai R, Miranda P, Valdẻs P, Lavin P,
Zepeda A, Casado ME, Silva MA, Fetis G, Bravo C, Chandia O, Peralta O, Croxatto
HB and Diaz S. Preregistration study on the safety and contraceptive efficacy
of a progesterone-releasing vaginal ring in Chilean nursing women.
Contraception 1999; 60(1): 9-14.
17. Massai R.
Quinteros E, Reyes MV, Caviedes R, Zepeda A, Montero JC, and Croxatto HB.
Extended use of a progesterone-releasing vaginal ring in nursing women: a phase
II clinical trial. Contraception 2005; 72(5): 352 – 357.
18. Contraceptive
Technology. Robert A Hatcher (ed); 17th Revised Edition, 1998;
Ardent Media Incorporated, page 410.
19. Holt VL,
Daling JR, McKnight B, Moore D, Stergachis A and Weiss NS. (1992) Functional
ovarian cysts in relation to the use of monophasic and triphasic oral
contraceptives. Obstet Gynecol 1992; 79: 529 – 533.
20. Turan C, Zorlu CG, Ugur M, Ozcan T, Kaleli B and Gökmen O. Expectant
management of functional ovarian cysts: an alternative to hormonal therapy. Int
J Gynaecol Obstet 1994; 47 (3): 257 - 260.
21. Nezhat FR, Nezhat CH, Borhan S and Nezhat CR. Is hormonal suppression
efficacious in treating functional ovarian cysts? J Am Assoc Gynecol Laparosc
1994; 1 (4, part 2): S26.
22. Westhoff C. Depot medroxyprogesterone acetate contraception.
Metabolic parameters and mood changes. J Reprod Med. 1996; 41 (suppl 5): 401 -
406.
23. Spencer JE. Anovulation and monophonic cycles. Ann N Y Acad Sci 1997;
16: 173 - 176.
24. Livingstone M and Fraser IS. Mechanisms of abnormal uterine bleeding.
Hum Reprod Update. 2002; 8 (1): 60 - 67.
25. Sharma JB, Roy KK, Pushparaj M, Gupta N, Jain SK, Malhorta N and Mittal
S. Genital tuberculosis: and important cause of Asherman’s syndrome in India.
Arch Gynecol Obstet 2008; 277 (1): 37 - 41.
26. Grivell RM, Reid KM and Mellor A. Uterine arteriovenous malformation: a
review of the current literature. Obstet Gynecol Surv 2005; 60 (11): 761 - 767.
27. Halperin R, Schneider D, Maymon R, Peer A, Pansky M and Herman A.
Arteriovenous malformation after uterine curettage: a report of 3 cases. J
Reprod Med 2007; 52 (5): 445 - 449.
28.Shapley M, Jordon K and Croft. An epidemiological survey of symptoms of
menstrual loss in the community 2004. Br J Gen Pract 2004; 54 (502): 359 – 363.
29. Schlesselman JJ. Oral contraceptives and
neoplasia of the uterine corpus. Contraception 1991; 43: 557 - 579.
30. Narod SA, Risch H, Moslehi R, Dørum A, Neuhausen S, Olsson H, Provencher
D, Radice P, Evans G, Bishop S, Brunet JS and Ponder BA. Oral contraceptives
and the risk of hereditary ovarian cancer. Hereditary ovarian cancer clinical
study group. N Engl J Med 1998; 339(7): 424 – 428.
31. The Cancer and Steroid Hormone Study of the Centres
for Disease Control and the National Institute of Child Health and Human
Development. The reduction in risk of ovarian cancer associated with oral contraceptive
use. N Engl J Med 1987; 316: 650 - 655.
32. Ness RB, Grisso JA, Cottreau C, Klapper
J, Vergona R, Wheeler JE, Morgan M, Schlesselman JJ. Factors related to
inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology
2000; 11: 111 - 117.
33. Mishell DR Jr. Noncontraceptive
benefits of oral contraceptives. Am J Obstet Gynecol 1982; 142: 809 – 816.
34.Brinton LA, Vessey MP, Flavel R, Yeates D.
Risk factors for benign breast disease. Am J Epidemiol 1981; 113: 203 – 214.
35.The ESHRE Capri Working Group. Non contraceptive
health benefits of combined oral contraception. Hum Reprod Update 2005; 11(5):
513 – 525.
36. Brunner Huber LR and Toth JL. Obesity and oral contraceptive failure:
Findings from the 2002 National Survey of Family Growth. Am J Epidemiol 2007;
1666: 1306 – 1311.
37. Curtis KM, Mohllajeea AP, Martinsa SL, Petersonb
HB. Combined oral contraceptive use among women with hypertension: a systematic
review. Contraception 2006; 73: 179 – 188.
38. Farley TMM, Collins J, Schlesselman JJ. Hormonal contraception and risk
of cardiovascular disease. An international perspective. Contraception 1998;
57: 211 – 230.
39. Lubianca JN, Faccin CS, Fuchs FD. Oral contraceptives: a risk factor for uncontrolled blood pressure among
hypertensive women. Contraception 2003; 67(1): 19 - 24.
40. Lubianca JN, Moreira LB, Gus M, Fuchs FD; Stopping oral contraceptives:
an effective blood pressure-lowering intervention in women with hypertension. J Hum Hypertens. 2005; 19(6):451 -455
41.Atthobari J, Gansevoort RT, Visser ST, de Jong PE
and de Jong-van den Berg LTW. The impact of hormonal contraceptives on blood pressure, urinary albumin
excretion and glomerular filtration rate. Br J Clin Pharmacol
2007; 63(2): 224 – 231.
42.Rees DC, Cox M and Clegg JB. World distribution of factor V Leiden.
Lancet 1995; 346: 1133 – 1134.
43.Blickstein D and Blickstein I. Oral contraception
and thrombophilia. Curr Opin Obstet Gynecol 2007; 19: 370 – 376.
44.No authors listed] Intrauterine
devices: an effective alternative to oral hormonal contraception. Prescrire
Int 2009; 18(101): 125-130.
45. Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on
carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev. 2009;
7(4): CD006133.
46. Cagnacci A, Ferrari S, Tirelli A, Zanin, Volpe A. Route of administration of contraceptives
containing desogestrel/etonorgestrel and insulin sensitivity: a prospective
randomized study. Contraception 2009; 80(1): 34 - 39.
47. Xiang AH, Kawoakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception
and risk of type 2 diabetes in Latino women with prior gestational diabetes
mellitus. Diabetes Care. 2006; 29(3): 613 - 617.
48. Shawe J, Lawrenson R. Hormonal contraception in women with diabetes
mellitus: special considerations. Treat Endocrinol. 2003; 2(5): 321 – 330.
49. Nikolov A, Dimitrov A, Kolarov G, Todarova K, Mekhandzhiev TS. Contraception in women with diabetes. Mellitus Akus Ginekol (Sofia). 2005; 44(5): 47 - 52.
50.International Collaboration of Epidemiological Studies of Cervical
Cancer. Appleby P, Beral V, Berrington de Gonzalez A, Colin D, Franceschi S,
Goodhill A, Green J, Peto J. Plummer M, Sweetland S. Cervical cancer and
hormonal contraceptives: collaborative reanalysis of individual data for 16,573
women with cervical cancer and 35,509 women without cervical cancer from 24
epidemiological studies. Lancet 2007; 370(9599): 1609 – 1621.
51.Wong MT, Singh K. The combined oral contraceptive pill in women
over age forty. Ann Acad Med Singapore 2003;
32(5): 624 - 631.
52.Singletary AE. Rating risk factors for breast cancer. Ann Surgery 2003;
237(4): 474 – 482.
53.Reid RL. Hormonal Contraception
and Breast Cancer: Keeping Perspective. J Obstet Gynaecol
Can 2007; 29(3): 207 – 209.
54. Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer:
a meta-analysis. Mayo Clin Proc 2006; 81(10): 1290 – 1302.
55.Liying Z, Bilian X. Emergency contraception
with Multiload CU-375sl IUD: a multicentre clinical trial. Contraception 2001;
64: 107 – 712.
56. Bhathena RK and Guillebaud J. Review
Postcoital contraception. The Obstetrician and Gynaecologist 2011; 13: 29 – 34.
57.Cameron S, Glasier A. The need to take a ‘new
look’ at emergency contraception. J Fam Plann Reprod Health Care 2010; 36: 3 –
4.
58.Brache V. Cochon L, Jesam C, Maldonado R,
Salvatierra AM, Levy D Gainer E, Croxatto HB. Immediate pre-ovulatory
adminstration of 30 mg ulipristal acetate significantly delays follicular
rupture. Hum Reprod 2010; 25(9): 2256 – 2263.
59.World Health Organisation. Emergency
contraceptive pills. In: Family Planning-A Global Handbook for Providers.
Geneva: WHO; 2007. P. 45 – 58.
60.Guillebaud J. Contraception for women over 35
years of age. Br J Fam Plann 1992; 17: 115 – 118.
61.Lidegaard O. Oral contraception and the risk
of cerebral thromboembolic attack: results of a case-control study. BMJ 1993;
306: 956 – 963.
62.Broome M, Fotherby K. Clinical experience with
the progestogen-only pill. Contraception 1990; 42: 489 – 495.
63.Wong MT and Singh K. The combined oral
contraceptive pill in women over age forty. Ann Acad Med Singapore 2003; 32(5):
624 – 631.
64. Kaunitz AM. Oral
contraceptive use in perimenopause. Am J Obstet Gynecol 2001; 185(2 Suppl): S32
– 37.
65.Lidegaard O, Lokkegaard E, Svendsen AL and
Agger C. Hormonal contraception and risk of venous thromboembolism: national
follow-up study. BMJ 2009; 339: b2890.
66.Hirvonen E, Idanpaan-Heikkila J.
Cardiovascular death among women under 40 years using low oestrogen oral
contraceptives and intrauterine devices in Finland form 1975 to 1984. Am J
Obstet Gynecol 1990; 163: 281 – 284.
67.Jick H, Jick SS, and Gurewich V, Myers MW and
Vasilakis C. Risk of idiopathic cardiovascular death and nonfatal venous
thromboembolism in women using oral contraceptives with differing progestogen
components. Lancet 1995; 346: 1589 – 1593.
68.WHO Collaborative Study of Cardiovascular
Disease and Steroid Hormone Contraception. Venous thromboembolic disease and
combined oral contraceptives: results of international multicentre case-control
study. Lancet 1995; 346: 1575 – 1582.
69.WHO Collaborative Study of Cardiovascular
Disease and Steroid Hormone Contraception. Ischaemic stroke and combined and
combined oral contraceptives: results of international multicentre case-control
study. Lancet 1996; 348: 498 – 505.
70.Gebbie A. Contraception in the perimenopause.
J Br Menopause Soc 2003; 9(3): 123 -128.
71.Alexander IM. Contraceptive options for
perimenopausal women. Contracept Technol Update 1999; 20(10): 122 – 124.
72.Bhathena RK and Guillebaud J. Contraception
for the older woman: an update. Climacteric 2006; 9()4): 264 – 267.
73.FHRPHC Guidance: contraception for women aged
over 40 years. J Fam Plann Reprod Health Care 2005; 31(1): 51 – 63.





