Chapter 7
Induction of Ovulation
Induction of ovulation is a term used to describe
stimulation of part or the whole of the hypothalamo-pituitary-ovarian (HPO)
axis by exogenous means to produce follicles and ultimately eggs. Normally the
ultimate objective is to develop one follicle, and to shed one oocyte to reduce
the risks of multiple pregnancies and hyperstimulation syndrome. Stimulation can
be at the level of the hypothalamus, pituitary gland, or directly at the level
of the ovaries. It is only indicated in women who are keen to conceive, but are
not ovulating regularly, or frequently enough to give them a good chance of
doing so. This is different from controlled ovarian hyperstimulation, when
multiple follicles are stimulated during an in vitro fertilisation treatment
cycle. The objective here is to produce multiple oocytes to be fertilised in
vitro, to create embryos which can be replaced into the uterus. Certain
conditions must be satisfied before induction of ovulation can be provided:
- The exact
cause of anovulation should be diagnosed, to allow proper selection of the
appropriate medication to stimulate ovulation. The indiscriminate
prescription of clomiphene citrate to all women with oligo-ovulatory
cycles without reaching a diagnosis is not appropriate.
- There should
be no medical or social reasons to contraindicate pregnancy. Medical
conditions should be addressed first, and social factors are better dealt
with in collaboration with a counsellor.
- At least one
fallopian tube should be patent.
- Specific
endocrine problems including thyroid and adrenal glands dysfunction, and
hyperprolactinaemia should be addressed first. Spontaneous ovulation may
restart without the need for active stimulation of the HPO axis.
- Obese and
underweight patients should be encouraged to regain an appropriate body
mass index between 18.5 – 24.9 kg/m2. This is more likely to kick-start
the HPO axis to resume functioning spontaneously. More information about
this subject can be found
in Chapter 8.
- There should
be no contraindication to use the specific drug selected.
- Monitoring
facilities and experienced medical supervision should be available to
maximise chances of patients’ safety. This will reduce, but does not
abolish altogether excessive ovarian response, ovarian hyperstimulation
syndrome or multiple pregnancies risks.
- Hospital
backup should be available to deal with any complications, especially
ovarian hyperstimulation syndrome.
Treatment rationale
Different drugs are available to work at different
levels of the HPO axis. They can be summarised as follows:
- Drugs acting
at the level of the hypothalamus [anti oestrogens];
- Drugs acting
at the level of the pituitary gland [pulsatile GnRH] ;
- Drugs acting
directly on the ovaries [gonadotrophins].
Disease rationale
The type of drug selected depends on the category of
the anovulation problem encountered. The WHO anovulation subgroupings will be used as examples in this case:
- Hypogonadotropic
hypogonadism [WHO type 1];
- WHO type 2
(non PCOS);
- Polycystic
ovary syndrome which makes more than 50% of all the
cases.
Aetiology of anovulation
Polycystic ovary syndrome has been described as the
most common endocrinopathy in women during their reproductive life (1). This
view has been shared by many other authors, and the following percentages of
endocrine dysfunctions have been reported in one series (2):
- PCOS was
diagnosed in 50.8% of all anovulatory women investigated.
- Hypothalamic
dysfunction was reported in 26.2% of the cases. Anovulation was linked to
weight related problems, psychological problems, or excessive physical
exercise. No specific association was noted in many patients.
- Ovarian
failure was reported in 8.7% of the patients as diagnosed by high FSH
blood levels. Induction of ovulation is not a viable option in these
patients.
- Hypo-or-hyperthyroidism
was diagnosed in 4.2% of all patients who presented with anovulation, or
anovulatory menstrual dysfunction. As stated before, treating the
underlying condition allows spontaneous resumption of ovulation in most
cases. Adequate ovulation may take longer time to resume after the
biochemical correction of the deranged thyroid indices.
- Secondary
amenorrhoea due to hypogonadotropic hypogonadism was
diagnosed in 4.0% of the cases mainly due to empty sella syndrome. The
diagnosis was made with the help of MRI.
- Hyperprolactinaemia
was reported in 3.1% of the cases.
- Different
other causes made the remaining 3.0%.
The exact percentages may be different in different populations,
and care should be taken to establish the correct diagnosis before commencing
patients on any specific medication.
Specific medications
Antioestrogens
Antioestrogens are the most commonly used drugs for
induction of ovulation. They are only effective in patients with anatomically
intact HPO axis, as in cases of PCOS and other types of WHO type 2 anovulatory
problems. They occupy oestrogen receptors at the level of the hypothalamus;
hence creating a false impression of central hypoestrogenic state. This will
induce the hypothalamus to produce more GnRH pulses to stimulate gonadotrophins
production by the pituitary gland. They are not effective in treating patients
with hypogonadotropic hypogonadism (WHO type 1), or hypergonadotrophic
hypogonadism (cases of ovarian failure) who already have
high blood FSH levels.
Clomiphene citrate (clomid) is the most widely used drug in this
group. It is a non-steroidal antioestrogen used for induction of ovulation
since 1961 (3). It has a long ½ life, so it occupies cytoplasmic oestrogen
receptors for a long period of time, rendering them unavailable after an
initial stimulation phase. Such downregulation of oestrogen receptors
ultimately creates a hypoestrogenic state, which the hypothalamus rectifies by
producing more GnRH pulses to increase gonadotrophins production. The
manufacturer’s product monograph reported that only 51% of an oral dose of
clomiphene citrate was excreted after 5 days, mainly in the stools. The
remaining part stayed in the body for a long period of time, mainly in the
enterohepatic circulation. Less than 1% of the drug was still being excreted
every day in faecal or urine samples collected 31 to 53 days after its
administration (4). This explains the protracted antioestrogenic effects of the
drug on the different systems of the body.
Since its discovery, various protocols have been
tried before settling with the classical 5 days courses. Treatment is usually
started after progestogen withdrawal bleeding. Extended therapy for 10 days has
been reported to be effective in patients who failed to ovulate after 5-day
courses. Fluker et al in 1996 (5) reported that 47% of initially resistant
patients ovulated after daily 100 mg doses, used between days 3-12 after
withdrawal bleeding episodes. They reported 65% of the treatment cycles to be
ovulatory in this group, with no significant side effects.
Ovulation usually occurs 7-10 days after the last
clomiphene citrate dose, but may occur a couple of days earlier. About 15-20%
of patients, mainly with PCOS, may not respond or only have an inadequate
response. Furthermore, only 30-40% of the patients who manage to ovulate usually
conceive (6), and the pregnancy rate is generally inversely related to the dose
used. Doses >100 mg/day are not usually indicated, and clomid should not be
used for more than 6 cycles (4). The discrepancy between ovulation rate and
pregnancy rate is related to its antioestrogenic effects, and reduced uterine
receptivity. Different detrimental effects have been reported following
clomiphene citrate therapy including:
- Thick and
non-watery cervical mucous reduces colonisation of the cervical crypts
with sperm, which is necessary for the continuous migration of sperm into
the uterine cavity.
- Patients on
clomid were found to have advanced histological maturation of the
endometrium relative to its chronological age.
- Changes in
fallopian tubes fluid chemistry and motility can affect the transport of
the sperm and / or embryos.
More recent studies showed reduced endometrial
thickness, and unfavourable endometrial pattern on ultrasound monitoring. This
was coupled by reduced uterine arteries, subendometrial and endometrial blood
flow in patients with PCOS using clomiphene citrate (7). Beside these specific
antioestrogenic effects, clomid has been shown to increase the blood level of
circulating androgens in patients with PCOS. This may have some bearing on the
quality of the eggs produced, and the receptivity of the endometrium. Some
women may also notice skin eruptions while on treatment. In fact all body
systems can be affected by clomiphene citrate, though significant systemic
complications are not common. The range of reported side effects included
headaches, low mood, nausea and vomiting, fever, hot flushes, tinnitus, chest
pain, shortness of breath, tachycardia, urticaria, arthralgia, back pain and
myalgia, among many others. More serious side effects included visual symptoms
including visual spots and blurred vision, due to intensification and
prolongation of the after-images in brightly lit areas. Diplopia, scotoma,
photosensitivity and reduced visual acuity have all been described. Treatment
should be stopped immediately, and not repeated in these cases. Changes in
liver enzymes and hepatitis have also been mentioned. It is important to
reiterate that systemic side effects are not common, despite this long list of
possible complications.
One of the more common side effects of clomiphene
citrate therapy is multiple pregnancies. This follows multiple follicular
recruitment and multiple ovulations in >30% of the patients. Ultrasound scan
follow up should alert the treating doctor to this possibility. These patients
should abstain from sexual intercourse during that cycle. Twin pregnancies have
been reported in 5-10% of all pregnancies following clomiphene citrate therapy,
but much lower figures were reported for twins (1 in 400 cases, 0.25%) and
triplets (1 in 800 cases, 0.12%).
Figure 24 shows monofollicular ovulation in a
patient with polycystic ovaries after induction of ovulation with clomiphene
citrate. A corpus luteum is seen in the right ovary, while the left one shows
polycystic changes. In contrast, Figure
25 shows a total of 11 recruited
follicles of different sizes.
Other side effects include:
- Clomiphene
citrate has a thermogenic effect, which may give
a false impression of thermal shift in non-responsive patients using
temperature charts to document ovulation.
- Premature LH
surge with small follicles is not uncommon
after clomiphene citrate induction of ovulation. Such occurrence can only
be detected if ultrasound monitoring is used.
- Increased
incidence of luteinized unruptured follicles (LUF) syndrome has also been
reported after using clomiphene citrate therapy. It can be diagnosed when
a follicle fails to collapse or to shows internal changes reminiscent of a
corpus luteum. Colour Doppler monitoring may show poor neovascularisation
around the luteinized follicle, and serum progesterone may be low as well.
The reported incidence of LUF ranged between 6-25% during the first cycle
of clomiphene citrate use. Higher percentages were reported in women with
unexplained infertility and regular cycles who received clomiphene citrate
during intrauterine insemination treatment cycles. Figures of 78% and 90%
were reported during the second and third cycles respectively in this
group of women (8). This detrimental effect on follicular rupture was
considered an indication for a possible role for clomiphene citrate in the
aetiology of LUF, either by central or local means.
Clomiphene citrate should not be used by patients with liver disease and ovarian cysts,
and by patients who had previous complications or hypersensitivity to the drug.
Accordingly, before prescribing any further doses of clomiphene citrate, care
should be taken to exclude the presence of undiagnosed pregnancy, and ovarian
cysts which follow 14% of the induced cycles. Such cysts are functional and
usually resolve spontaneously. It is also contraindicated in women with
undiagnosed vaginal bleeding, and for the management of menstrual disorders in
women who are not keen to get pregnant.
Other drugs have been combined with clomiphene
citrate to improve its efficacy including corticosteroids, bromocripine, as well as oral and injectable
oestrogens. Many studies have reported the efficacy of these medications in
improving the response of patients who were initially resistant to clomiphene
citrate. Pre-treatment with an oral contraceptive pill, and combined treatment
with oestradiol benzoate injections (9), corticosteroids (10), bromocripine,
and human chorionic gonadotrophin for the final triggering of ovulation have
all been used with different claims of success, after an initial poor response.
On the other hand, a Cochrane review published in 2009 showed no extra benefit
of adding human chorionic gonadotrophin to clomiphene citrate (11).
Nevertheless, the same review showed improved pregnancy rate when clomiphene
citrate was combined with dexamethasone, or followed a course of oral
contraceptives.
Other antioestrogenic drugs have also been used for
induction of ovulation. The most commonly reported one is tamoxifen, which can
be given in a dose of 10 – 20 mg every day for 5 days, starting on day 3 of a
progestogen withdrawal bleeding. It is not very popular in
comparison to clomiphene citrate. Recently, aromatase inhibitors have also been documented as effective drugs
for induction of ovulation, either separately, or in combination with
clomiphene citrate. By inhibiting the aromatase enzyme, they reduce the
conversion of androgens to oestrogens, and hence create a hypoestrogenic
effect. This can lead to increased production of GnRH pulses by the
hypothalamus and gonadotrophins by the pituitary gland. The most commonly used
drug in this group is letrozole which is a third generation aromatase
inhibitor. It is given in a dose of 2.5 mg/day for 5 days, starting on day 3 of
a progestogen withdrawal bleeding as well. A single dose of 10 – 30 mg was
found to be equally effective as the 5-day course. Letrozole has been shown to
be effective in clomiphene citrate resistant cases. It has another advantage of
a short half life of 45 hours compared to the very long half life of clomiphene
citrate which could occupy nuclear oestrogen receptors for 6-8 weeks.
Accordingly, it is less likely to have significant detrimental effects on the
cervical mucous and endometrium. Letrozole has also been used with
gonadotrophins for induction of ovulation in poor responders with some good
effect. It may also be used to augment the effect of gonadotrophins, hence
reducing their total dose for cost saving. Other drugs within this group
include anastrozole and exemestane. All aromatase inhibitors have
similar antioestrogenic side effects as clomiphene citrate. They are not yet as
popular as clomiphene citrate, but are potentially useful for patients who can
not take clomiphene citrate, and in resistant cases (12).
There is some controversy regarding the value of
ultrasound monitoring after using antioestrogens induction of ovulation, mainly
clomiphene citrate. Unavailability of the service and the cost of repeated
ultrasound scan examinations were the main factors responsible for the
historical use of clomiphene citrate without monitoring. Many of the benefits
in following patients with serial ultrasound scan examinations before and
during clomiphene citrate medication have been discussed before, including:
- To avoid taking
the drug in cases with residual corpus luteal cysts. This is especially
important during follow up cycles after previous use of the drug. Such
cysts were reported in 14% of the cases as mentioned before;
- Assurance of
follicular growth as ≈20% of the
patients may not respond;
- To detect
multiple follicular development with increased risk of multiple ovulation
(30 %) and multiple pregnancy (5 – 10%);
- To detect ovulation
of small follicles due to premature LH surge;
- To confirm
ovulation which is necessary for selecting the optimum time for
intercourse or artificial insemination;
- To detect
cases with LUF syndrome (25 – 90%) as discussed to before.
One drug which gained great notoriety is metformin (glucophage), which is an insulin sensitising
biguanide drug, used for the treatment of type II diabetes mellitus. It had an
equal effect in improving anovulatory menstrual irregularities in both insulin
resistant and insulin sensitive PCOS patients (13). A direct effect on the
ovaries has been reported even in women who were not insulin resistant (14). A
good account has been given about its mode of action, dose, and side effects in
Chapter 6. It proved effective in the treatment of anovulatory PCOS patients by
inducing regular menstrual cycle and fertility. It was also effective in
converting clomiphene citrate resistant patients into responsive ones (15), and
reduced early miscarriage rate (16, 17). The usual dose is 500 mg twice daily
with food to reduce gastrointestinal side effects. It also has a favourable
local effect at the level of the uterus, besides its other favourable endocrine
effects on hyperinsulinaemia and hyperandrogenaemia. Such effects include
improved endometrial thickness as well as uterine, subendometrial and endometrial
blood flow to levels usually seen in a non PCOS control groups (18). It is
inevitable that many patients on metformin, which is classified as category B
drug for use during pregnancy, will fall pregnant while taking the drug. Recent
work showed that it reduced the development of gestational diabetes in women
with PCOS (19), and was safe to use during pregnancy (20).
Gonadotrophins
Human pituitary gonadotrophins were isolated in 1958,
and utilised successfully for induction of ovulation. They have been withdrawn
from the market because of their limited supplies, and the association of
Creutzfeldt-Jacob disease with human pituitary growth hormone. Products
prepared from postmenopausal women urine have been used since the 1960s, and
recombinant products were the latest to be produced. This incurred major
changes in prices for patients, in return for more pure products and stable
batch to batch consistency. Nonetheless, no significant differences in odds of
pregnancy outcome or complications have been found between urinary and
recombinant FSH during induction of ovulation in patients with PCOS in a
Cochrane review conducted by Bayram et al in 2003 (21). The situation was
rather different during assisted reproduction treatment cycles in a different
Cochrane review conducted in the same year by Daya and Gunby (22). They
reported significant increase in the odds of clinical pregnancy and live birth
or ongoing pregnancy for recombinant FSH versus urinary FSH. This view has been
challenged by a more recent randomised controlled multicentre study published
by Abate et al in 2009 (23). They found no difference in oocyte or embryo
quality produced by patients using either medication. Furthermore, there was no
difference in the fertilisation, cleavage and implantation rates, or pregnancy
and miscarriage rates between the two groups. Interestingly, less urinary FSH
was needed for a shorter period of time than the recombinant FSH during the
same study to give a similar clinical response. Combined drugs with equal
proportions of FSH and LH, or only FSH medication are available in the market.
Despite many claims and counter claims in the past, it is now evident that
there is no significant superiority of one product over the others, in relation
to induction of ovulation or pregnancy rate.
Gonadotrophins are currently used as primary
medication in WHO group 1 and clomiphene citrate resistant patients. They act
directly on the ovaries, with about 20% chance of multiple pregnancies. Hyperstimulation occurs despite
strict monitoring, as follicles are produced in sequence rather than in
parallel. It is usually advisable to start with the smallest dose, and to step
it up as necessary. Doses and response may differ in different cycles, even in
the same patient; hence the need for strict monitoring. Pre treatment
counselling is necessary regarding the need for repeated visits to the clinic
for monitoring, and to discuss the risks of hyperstimulation and multiple
pregnancies. The need to cancel a cycle should be stressed clearly to the
patient, in case of excessive response. Smaller doses should be used in the
subsequent cycles.
Gonadotrophins dosage
One should always aim at monofollicular ovulation
taking into account the endogenous FSH. Patient with WHO group 1 usually need
higher doses, with lower risk of OHSS than women with PCOS or PCO. Treatment
should be started with one ampoule of the preferred gonadotrophin for 5 – 7
days, and the dose should be increased by half or one ampoule every 5 - 7 days,
if necessary. The minimum effective dose should be maintained till a mature
follicle has developed. With excessive recruitment, the dose should be stepped
down, or even abandon the cycle if multiple follicles have already developed.
The minimum effective dosage should be used in subsequent cycles.
Regular monitoring with ultrasound scanning is
adequate without the need for oestradiol estimations, in most if not all cases.
If oestradiol is used, the ideal daily rise in serum level was shown to be a
factor of 1.3 - 1.4, as seen during natural cycles. Exponential rise indicates
increased risk of excessive response and hyperstimulation. Follicles may be
recruited and grow in sequence, rather than in parallel in response to
gonadotrophins medication during controlled ovarian hyperstimulation.
Accordingly, new follicles may be seen each time a scan examination is
preformed. A folliculogram is best suited to show this response as follicles
would be spread along the whole column, rather than being grouped together as
siblings of similar size. There is a higher chance of multiple follicles development
with gonadotrophins (>60%), in comparison to clomiphene citrate (>30%).
It is also noticeable that different patients hyperstimulate at different
oestradiol levels. Accordingly, ultrasound scanning is a better parameter than
E2 in predicting multiple pregnancies and OHSS. This depends on the total number of
stimulated follicles, especially intermediate size ones, as will be discussed
later on in this chapter.
The primary objectives for monitoring ovulation are:
- To secure
patients’ safety by detecting early signs of excessive response which
precedes hyperstimulation;
- To evaluate
patients’ response to therapy in an attempt to secure monofollicular
ovulation whenever possible;
- To document
changes in the endometrial thickness and echotexture;
- To time hCG
injection to trigger ovulation;
- To document
ovulation has taken place.
It has been established that all these requirements
can be fulfilled using ultrasound monitoring. This is because of the close
relationship between follicular development and endometrial changes as
documented by ultrasound on one hand, and oestradiol level on the other.
Furthermore, ultrasound scanning can be used to time the hCG injection, whereas
oestradiol is not useful in this respect. It can be useful for withholding the
hCG injection, if the blood tests showed exponential rise in oestradiol levels.
The reason why hyperstimulation occurs at different oestradiol levels depends
most probably on the maturation index of the follicles at the time of the hCG
injection. Monitoring ovulation should begin before starting medication to rule
out the presence of ovarian cysts or any other pathology. The following scan
should be performed 5-7 days after starting medication. The number of recruited
follicles should be counted, and marked in a specially designated
folliculogram. This should be filled serially, as it gives a visual picture
regarding the number and the rate of follicular growth. The maximum endometrial
thickness should be measured in the sagittal plane, during each examination.
Its texture pattern should also be noted, being isoechoic to the myometrium,
hypoechoic or echogenic. A trilaminar pattern with hypoechoic texture is
considered to be the most receptive.
Figures 26 – 28 represent thin menstrual,
midcycle trilaminar, and echogenic secretory endometrium respectively. Cervical
mucous can also be seen as a dark line occupying the cervical canal in Figure 27.
Ovulation can be predicted biochemically by measuring
the LH surge. This can be done by urine
prediction kits or by serial measurements of LH blood levels, once a follicle
has reached 16 mm in diameter. Neovascularisation of the dominant follicle, as
detected by colour Doppler mapping, is a good predictor of imminent ovulation
as well. All this might be academic, as an exogenous hCG injection is usually
given to help with ovulation and timed intercourse. A recent study published by
Farhi et al in 2010, showed that pregnancy rate was affected by follicular size
when hCG was administered to trigger ovulation (24). The rate was highest (13.6
– 18.6%) when the follicles were 18-22 mm in diameter and lowest with 17 mm
(8.8%), 23 mm (8.8%), and 24 mm (5.7%) follicles respectively. Most important,
there was no difference in pregnancy rate between cycles with one or two
follicles. In general, serum progesterone estimation one week following
ovulation helps to indicate adequate ovulation or otherwise. Different levels
have been used, but in general a blood level ≥30 nmol/l is considered to
reflect adequate ovulation. On the other hand, ultrasound scanning can show the
following signs of ovulation:
- Collapse of
the monitored follicle;
- The follicle
becomes smaller with thicker wall;
- The follicle
outline becomes irregular with the appearance of intra-follicular echoes
due to the presence of blood clots and serum;
- There is
increase in the amount of fluid in POD;
- The
endometrium shows an echogenic texture.
The corpus luteum has different looks depending on
the amount of haemorrhage into the sac itself, and the time lag between
ovulation and transvaginal ultrasound scan examination. Figure 29 shows a solid corpus luteum shortly after ovulation. A
blood clot occupies the whole sac. Figure
30 shows a corpus luteum with a blood clot and irregular haemolysed areas
in the middle, few days after ovulation. Figure
31 depicts a corpus luteum with a resolving blood clot showing reticular
texture, may days after ovulation. In general, a corpus luteum behaves like any
other haematoma as related to the texture, resolution and disappearance of the
blood clot.
Occasionally a follicle may fail to ovulate despite
increased oestradiol level, occurrence of an LH surge and rise in the level of
luteal phase serum progesterone. This can occur in cases of luteinized
unruptured follicle syndrome (LUF), which is seen more common in infertile
women. In such cases, ultrasound scan examination may show a persistent
follicle after the LH surge, with minimal vascular markings of the luteinized
follicle during colour Doppler mapping. In the pre-ultrasound era, diagnostic
laparoscopy was the main tool to diagnose LUF through failure to see ovulation
ostium, and low serum progesterone in the peritoneal fluid during the luteal
phase of the cycle. In cases of premature LH surge, ultrasound scanning can
show ovulation of a small follicle with a thin endometrium. This can occur
during both natural and clomiphene citrate induced cycles.
Figure 32 shows a new
ovulation ostium, whereas figure 33
shows a corpus luteum with evident yellow discolouration, both in the left
ovary in different women.
The role of Doppler Ultrasound
Doppler ultrasound has been studied extensively in relation to
the uterine, endometrial and ovarian vasculature during spontaneous and induced
ovulation. The uterine artery had more dominance in these studies, and basic
findings can be summarized as follows:
- The uterine
arteries can be identified just lateral to the cervix;
- Typically
they have moderate to high blood flow velocity;
- Their resistance
index depends on the phase of the cycle;
- They have
small end diastolic flow during the proliferative phase;
- The
resistance index declines before ovulation, and stays low till
menstruation.
Basic Doppler studies of the ovarian arteries can be
summarized as follows:
- Ovarian
vessels can be seen lateral to the upper pole of ovaries;
- They are
more difficult to visualize than the uterine arteries;
- They do not
show prominent colour flow on colour Doppler mapping;
- They
typically have low velocity;
- Their resistance
index depends on the phase of cycle.
Doppler studies at the middle of the cycle can show
the following characteristics:
- Reduced
resistance index of the uterine arteries and increased vascularisation of
the dominant follicle in normal cycles;
- Low or absent
subendometrial and endometrial perfusion in infertile women;
- Absent
uterine arteries diastolic flow in some infertile women.
Certain sonographic parameters have been considered
as favourable when documented during induction of ovulation:
- An endometrial
thickness ≥8 mm;
- A hypoechoic
endometrium with trilaminar echotexture;
- A dominant
follicles 18-22 mm in diameter (24)
- Uterine
artery pulsatility index < 3;
- High degree
of endometrial blood perfusion as shown by colour Doppler or 3D power
Doppler histograms reflect favourable endometrial receptivity (25).
Figure 34 shows a midcycle
transvaginal ultrasound sagittal view of a uterus with thick endometrium and
good endometrial and subendometrial blood flow, as shown by colour Doppler
mapping. Figures 35 demonstrates a
mature follicle with peripheral colour markings revealed during colour Doppler
examination, and figure 36 shows
favourable uterine artery Doppler pulse waveform, representing both the
systolic and diastolic parts. The pulsatility index was 2.05 as shown in the
lower right corner by the electronically generated data. Absence of the early
part or the whole diastolic pulse had been associated with increased impedance,
low tissue perfusion and infertility (26, 27).
Triggering ovulation with hCG injection
It is usual to trigger ovulation with an exogenous
dose of hCG, rather than wait for a spontaneous LH surge. This can be a necessity in the
following conditions:
- For patients
with hypogonadotropic hypogonadism;
- Following
pituitary downregulation with a GnRH-analogue;
- For better
timing of ovulation in cases of intrauterine insemination;
- When a
follicle has reached 20-22 mm in diameter without evidence of an LH surge,
or a good colour rim demonstrable by colour Doppler mapping.
The usual hCG doses used for triggering the final act
of ovulation are 5000 and 10000 IU, and ovulation usually occurs 36-40 hours
later. The ovulation window can last for 3 or more days, as follicles of
different sizes may take different times to ovulate. Secondary follicles
continue to grow for a day or more with the production of more oestradiol,
before ovulating. This group is the one more likely to cause OHSS, and multiple
pregnancies. Smaller follicles usually
luteinize immediately after hCG, and become atretic. The drug may stay in
circulation for up to 10 days after a dose of 10000 IU. So it can trigger
ovulation, and maintain luteal support. Such a high dose is more likely to have
a wide ovulation window, so it is not ideal for patients with multiple
follicles. Accordingly, a dose of 5000 IU should be used to trigger ovulation,
and to reduce the risk of OHSS. Similarly, luteal hCG injections to support the
corpus luteum can also increase the risk of OHSS. When necessary, progesterone
supplements will be a better option, and should be used instead.
The triggering dose of hCG affects rupture of the
dominant follicle through different mechanisms:
- It increases
follicular fluid volume;
- It also increases
collagenase and plasmin activity;
- It increases
the level of prostaglandins F2a;
- It increases
the contractility of myoepithelial cells in the ovary.
At the same time, the triggering hCG injection
affects oocytes maturation. Normally oocytes meiosis is inhibited by the enzyme
oocyte maturation inhibitor (OMI) produced by the granulosa cells. This
OMI does not act directly on the oocytes, but through the cumulus cells. It is
inhibited by the natural LH surge or hCG injection used to trigger ovulation of
a mature follicle. They cause withdrawal of the cumulus cell mass, resulting in
break down of the cumulus cells-oocytes communication.
A different role for hCG has been reported within the
setup of controlled ovarian hyperstimulation protocol which is used during
assisted reproduction treatment cycles. Small daily doses of 200 IU were found
to support growth and maturation of follicles >12 mm in diameter, without
any detrimental effects on the quality of the follicles. Such medication was
associated with reduced number of small preovulatory follicles, resulted in
more oestrogenic intrafollicular environment, and reduced FSH/HMG consumption (28)
Induction of ovulation with pulsatile GnRH
GnRH is a decapeptide produced by the hypothalamus.
It can be used in cases of hypothalamic failure or dysfunction. The best
results should be expected in women with WHO type 1 anovulation
[hypogonadotropic hypogonadism]. It is given through a pulsatile pump in a dose
of 5-15 µg/pulse, every 90 minutes. Smaller doses are used when the intravenous
route is used, rather than the subcutaneous one. It has the least risk of
producing OHSS or multiple pregnancies, which should be taken into
consideration when weighing its advantages and disadvantages.
Usually an exogenous hCG injection is not necessary
to trigger the final act of ovulation, as a spontaneous LH surge usually
occurs. Furthermore, luteal support can be maintained with the same pulse dose
of GnRH. Alternatively, an exogenous progestogen can be used, to give the
patient a break from continuous use of the pump.
The drawbacks of using pulsatile GnRH for induction
of ovulation include:
- Treatment
can only be given within a dedicated infertility unit with staff available
to respond to patients’ problems;
- Patients
must be motivated with good sense of hygiene to reduce the risk of
infection;
- The needle
site should be changed regularly. This should be done weekly for subcutaneous
sites, to avoid infection. More frequent changes will be needed with the
intravenous route of administration, depending on dislodgment of the
needle, or appearance of local signs of phlebitis;
- The cost of
the pump and the dedicated type of needles should be taken into
consideration;
- It may take
longer to respond to pulsatile GnRH medication than to gonadotrophins
depending on the dosage.
Ovarian electrocautery for induction of ovulation
Laparoscopic ovarian electrocautery is useful as a second line management for
patients with PCOS not responsive to clomiphene citrate therapy. It is most
effective in patients with blood LH levels > 12 IU/L, as reported by
Abdel-Gadir et al in 1993 (29). Both LH and testosterone were reduced after the
procedure, and it was equally effective as gonadotrophins for ovulation
induction, as reported by the same authors. Almost 50% of the conceptions
occurred within 6 months in patients with no other fertility problem.
Nevertheless, about 25% of the patients did not respond, but got more sensitive
to clomiphene citrate. A recent study reported by Amer et al in 2009 (30) found
that patients with high AMH level ≥ 7.7 ng/ml had reduced chance of ovulation
after the procedure. This can be used as another parameter, beside LH, to
counsel patients regarding chances of response before surgery.
Ovarian drilling has many merits in comparison to
other methods of induction of ovulation. It is a simple procedure which is easy
to learn, and does not need strict ultrasound or oestradiol monitoring. There
were also no increased risks of OHSS or multiple pregnancies, as documented in all the reports
published so far. Many patients will continue to have regular periods for a
long time, without the need for any further medication. Nonetheless, there is a
danger of misusing the technique in patients who are not seeking to get
pregnant. There is also the risk of ovarian adhesions if the technique used is
not perfect. Using microsurgical principles can reduce this risk (31). The
exact details have already been described in Chapter 6.
|
|
|
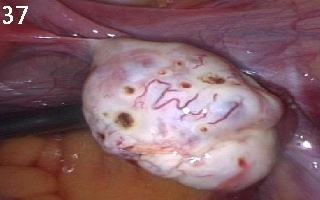
Figure 37 shows a
polycystic ovary after ovarian drilling. Note that there was no slit
cauterisation. Also note the characteristic dilated blood vessels usually
seen on the surface of polycystic ovaries.
|
The risk of primary ovarian failure has been raised,
but never materialised in any prospective study. Ovarian reserve markers were
found to be lower after ovarian drilling, compared to women with PCOS who did
not have the same procedure (32). The changes in FSH, inhibin B and antimullerian hormone blood levels, which
were used as measures of ovarian reserve, should be considered as signs of
normalisation of ovarian function rather than a reduction of ovarian reserve as
suggested by Murat in 2009 (33). It is understandable that over cauterisation
of the ovaries can lead to non-reversible damage. Accordingly, the number of
drills should always be guided by the size of the ovary itself, and the
duration of the current application should not exceed 4 seconds each time a
drill is made.
Induction of ovulation in women with regular periods
Induction of ovulation in women with regular cycles
raises many medical and ethical questions. This is especially so as drugs meant
to stimulate the ovaries can unnecessarily lead to multiple pregnancies and OHSS. They may not improve the fertility
potential in women <35 years, but can increase the multiple pregnancy rate.
On the other hand, increasing the number of follicles in older women with
gonadotrophins injections can increase cycle fecundity rate. This is usually
done within a protocol of timed intercourse or intrauterine insemination (IUI).
Nevertheless, the delivery rate in women over 40 years of age was less than 5%,
following gonadotrophins injections and IUI as reported by Tsafrir et al in
2009 (34) Furthermore, induction of ovulation is not beneficial and should be
avoided in patients with regular cycles and high FSH blood levels. There is
always a risk that unnecessary use of clomiphene citrate in regularly cycling
women may reduce cycle fecundity, due to its antioestrogenic effects on the
cervical mucous, endometrium and fallopian tubes as mentioned before.
Furthermore, there is increased risk of LUF (8).
Early pregnancy scanning
Following adequate ovulation and a positive pregnancy
test, early pregnancy ultrasound monitoring should concentrate on the following
points:
- Confirm a
diagnosis of intrauterine pregnancy;
- Exclude the
possibility of an empty uterus with positive ßhCG;
- Exclude or
ascertain a diagnosis of multiple pregnancy;
- Ascertain
chorionicity of multiple pregnancies. A thick
septum between the two sacs during early pregnancy usually indicates
binovular twining. The lambda sign is useful in this respect in the second
trimester;
- Help with
monitoring of disturbed pregnancies;
- Can be used
for embryo reduction in cases of higher order multiple pregnancies.
Ovarian hyperstimulation syndrome
Ovarian hyperstimulation syndrome is the most serious complication to follow
induction of ovulation, and may lead to significant morbidity or even mortality.
It is characterised by marked ovarian enlargement, high serum sex steroids, and
extravascular exudation of fluid and protein due to increased vascular
permeability. This can result in intravascular volume depletion,
haemoconcentration, diminished organ perfusion and increased thrombotic
tendency (35). It is usually more common with gonadotrophins than clomiphene
citrate induced cycles (36). Paradoxically, it is more common with induction of
ovulation than with superovulation utilised during IVF treatment cycles, as the
risk is reduced by follicular aspiration during oocytes retrieval. Certain
patients are more at risk than others, and the following factors increase the
risk independently as reported by the Practice Committee of the American
Society of Reproductive Medicine (36):
- Young age;
- Polycystic
ovaries;
- Low body
weight;
- Higher doses
of exogenous gonadotrophins;
- High
absolute or rapid rise in serum oestradiol;
- Previous
episodes of OHSS;
- Exogenous
hCG injections to trigger ovulation;
- With hCG
injections for luteal support;
- During
conception cycles because of endogenous hCG production.
It is generally noticeable that a patient’s
characteristics determine her individual response, more than the stimulation
protocol. Of all these risk factors polycystic ovaries stand out as the most
important entity. The risk is also higher during the first treatment cycle, and
in patients with hypothyroidism and hyperprolactinaemia. It is also higher in downregulated
cycles. GnRH analogues increase the risk by blocking the spontaneous LH surge
and luteinisation which are the self protecting mechanisms against further follicular
development (37). At the molecular level, it has been shown Mayorga et al (38)
that ovarian response to FSH stimulation depends on the patient’s FSH receptor (FSHR)
genotype. The same authors suggested that the number of FSH ampoules needed by
each patient could be predicted from a linear combination of basal FSH level
and the type of FSHR polymorphism.
The exact incidence of OHSS is usually difficult to
ascertain, because of under reporting, and the different diagnostic criteria
used. Furthermore, mild forms may even pass unnoticed or unreported by the
patients themselves. A figure of 4% has been reported after standard ovulation
induction with gonadotrophins with < 1.0% in its severe form. The overall
incidence of moderate and severe OHSS during IVF cycles was reported by
Brinsden et al in 1995 (39) as 1-10%, but only 0.5 – 2% of the cases were in
the severe form. A figure of 38% was quoted by Asch et al in 1991 (40) when
oestradiol level exceeded 10,000 pmol/l on the day of hCG administration.
Furthermore, the higher the number of eggs collected, the higher was the risk.
This risk exceeded 20% when 30 oocytes were collected, as reported by the same
last authors. Different oestradiol cut off figures have been quoted when the
risk of OHSS increased. In contrast, the rate of increase in oestradiol blood
levels, rather than the absolute values, has been reported to be more important
in this respect (37). This could reflect the hypersensitivity of the ovaries to
stimulation. At the same time, the value of oestradiol levels in predicting
OHSS has been questioned in different reports (41, 42). Accordingly, a
combination of different parameters should be used during monitoring, including
the following:
- The number
of follicles;
- The rate of
rise in oestradiol level;
- The actual
level of oestradiol;
- The ratio of
mature vs. intermediate follicles which denotes follicular maturation
index on the day of hCG adminstration.
Patients with more secondary than tertiary follicles
are more at risk to develop OHSS. This was demonstrated by a report published
by Blankstein et al, as far back as 1987 (43). In mild OHSS, 68.7% of the
follicles were 9 – 15 mm in diameter. On the other hand, 95% of the follicles
were <16 mm in diameter, most of them (54.7%) <9 mm in cases of moderate
to severe OHSS. This last point can explain the different oestradiol levels
reported in the literature when the risk of OHSS increased, without taking note
of the ratio of the number of secondary and tertiary follicles.
|
|
|
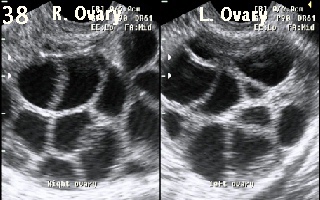
Figure 38 shows
excessive ovarian response during IVF treatment cycle. More than 10 follicles
were recruited in each ovary. The cycle was cancelled to prevent the
development of OHSS.
|
It seems that certain women are more liable to
develop OHSS than others. Figures 39 – 41 belong to a patient who developed
moderate OHSS with ultrasonically diagnosed ascites during an IVF treatment
cycle, in spite of normal ovarian response and normal oestradiol blood levels.
Furthermore, despite the presence of significant ascites, her blood chemistry
was not significantly affected. Figures
39 and 40 show transabdominal
pictures of the right and left ovaries with only few residual corpus luteal
cysts. Excessive amount of fluid is visible around the right ovary and uterus
as shown in figure 40. The right and
left ovaries were only mildly enlarged with diameters of 6.8 x 4.8 cm and 7.2 x
5.1 cm respectively. Figure 41 shows
some fluid under the diaphragm and on top of the liver. This case shows that
ovarian size, blood chemistry and the presence of significant ascites may not correlate
in the same patient.
The exact cause of OHSS is not known, but certain
factors were implicated in the development of OHSS, and have been summarised as
follows (36):
- High
follicular fluid level of proteins and renin;
- Angiotensin
mediated increased capillary permeability;
- Excessive
exudation of protein-rich fluid from the peritoneal surface and enlarged
ovaries.
Though only the ovarian protein-renin-angiotensin system
is mentioned in this list, other ovarian vasoactive factors are also involved
in mediating increased capillary permeability. The list includes the kinin
kallikrein system, selectins, von Willebrand’s factors, prolactin, prostaglandins,
but the most important one is vascular endothelial growth factor (VEGF) (37).
This was shown by a major impact of recombinant VEGF antiserum in neutralising capillary
permeability activity (44, 45). Further work showed significantly high free or
unbound VEGF, and lower plasma and follicular fluid levels of the corresponding
binding protein in patient with OHSS (46, 47). On the other hand it, is has
been suggested that activation of the renin-angiotensin system in patients with
OHSS may be a secondary response rather than a primary factor in the
pathogenesis of OHSS (37).
The syndrome typically starts approximately one week
after ovulation, but the affected patients may not show any specific symptoms
to start with. The spectrum of abnormalities ranges from ovarian enlargement,
to severe multiple systems failure. Patients may present with abdominal pain,
feeling bloated and tired, diarrhoea and thirst, and abdominal distension in
the early stages. In most cases the condition is self-limiting, and strict
observation and reassurance will be adequate. The range of severe clinical and
biochemical problems can be grouped into:
- Volume
depletion, hyponatraemia and ascites;
- Haemoconcentration
& thrombotic tendency;
- Renal and
hepatic dysfunction and respiratory distress.
For such a self-limiting problem, unnecessary
intervention can cause more harm than benefit. Accordingly, the management plan
should focus on the following points:
- Proper
assessment of the severity of the condition, bearing in mind its dynamic
nature which can change from one day to another;
- Provision of
symptomatic relief and reassurance to the patient;
- Avoidance of
haemoconcentration;
- Prevention
of thromboembolism;
- Maintenance
of cardiorespiratory and renal function.
These are easy objectives to set, but can be very
taxing in cases with severe OHSS. Different methods have been used to classify
its severity since 1967, when the first classification was introduced by Rabau
et al (48). Various clinical, ultrasound and biochemical parameters have been
combined into many subgrades which made them rather difficult to use. For
clinical purposes, the following simplified classification has been used in
many fertility units for many years, with good effect:
- Mild OHSS
when the ovaries are < 8 cm in diameter, and the patient complains of
abdominal bloating, heaviness and mild pain;
- Moderate
OHSS entails ovaries 8-12 cm in diameter with increased abdominal
discomfort, nausea and vomiting, and ultrasound evidence of ascites;
- In severe
cases of OHSS, the ovaries exceed 12 cm in diameter, with clinical
evidence of ascites, hypovolaemia, haemoconcentration, electrolyte
imbalance, decreased renal perfusion and liver dysfunction. All these
systems dysfunctions need regular blood chemistry assessments to determine
their severity and progression. The ascites can be tense, and there may be
evidence of hydrothorax and generalised oedema.
Not all patients with OHSS need daily hospital
supervision, or admission to hospital. These will incur unnecessary
inconvenience and expenses to the patients. In mild cases, outpatient
management entails adequate oral fluid intake, at least 1 litre per day. The
patient should also weigh herself daily, and any excessive weight gain of more
than 2 pounds per day should be reported to the hospital for further
investigations. She should also observe her own urine output. Any reduction in
daily output or passage of concentrated urine should likewise be reported to
the hospital. Furthermore, strenuous exercise should be avoided to prevent
trauma or torsion of the enlarged ovaries. It is advisable to see the patient
every few days to measure her abdominal circumference at a fixed point each
time, and to ascertain her general condition. In cases of moderate to severe
OHSS, the major cardinal monitoring parameters should include:
- Signs of
dehydration and fluid retention with deranged blood electrolytes;
- Haematological
signs of haemoconcentration including PCV > 50% and WBC >25 X 103;
- Signs of
decreased renal perfusion and renal failure, as shown by decreased urine
output and changes in blood chemistry;
- Signs of
liver dysfunction with deranged liver function tests;
- Signs of
adult respiratory distress syndrome which may be difficult to diagnose
initially. The patient may present with rapid pulse, shortness of breath
with no abnormal signs on chest examination. The condition may deteriorate
fast, and the patient could become hypoxic or even cyanotic, and in need
of positive pressure ventilation. Arterial blood gas assessment is
indicated in hypoxic patients at risk even in the absence of physical
signs.
Management of moderate and severe OHSS
Strict initial clinical, ultrasound and biochemical
assessment will give a clear picture of how much a patient’s systems are
deranged. This helps regarding further management plans, and whether the
patient should be referred to a unit with intensive care facilities. It is
important to bear in mind that the general clinical appearance and the extent
of ovarian enlargement are not good indicators of the patient’s biochemical
derangement. The following guidelines should be followed:
- Aim at
reassurance to reduce anxiety;
- Keep a
strict fluid chart and start crystalloid solutions 100-150 ml/ hour, if
the urine output < 400 ml/day or the PCV > 45%;
- Add low salt
human albumin in a dose of 100 gm every 3-12 hours by intravenous
infusion, if the PCV has not improved or the patient has developed
oliguria;
- To reduce
the risk of deep venous thrombosis, patients should be encouraged to use
full length thrombo-embolic deterrent (TED) stockings, and to mobilise as
soon as possible;
- Subcutaneous
heparin in a dose of 5000 IU twice/day should be used to guard against
thromboembolic problems;
- A diuretic
(furosemide) should be used only if PCV has improved, but there is no
diuresis yet. Otherwise, it should be avoided as it can lead to further
dehydration of the intravascular compartment and electrolyte derangement;
- Dopamine
infusion can be used in a dose of 5 µg / kg body weight / min, in patients
with impending renal failure despite implementation of all the above
measures;
- Patients
with tense ascites which is interfering with breathing should have
ultrasonically guided paracentesis of the peritoneal fluid. This will
reduce its splinting effect on the diaphragm, and can help with urine
output as well in patients suffering from oliguria. This fluid is very
rich in plasma proteins, and can lead to proteins deprivation if large
amounts of the ascitic fluid are removed.
It is important to remember
that OHSS is a self-limiting condition, and resolves spontaneously in the
majority of cases. Accordingly, treatment should fundamentally be supportive.
More damage can be incurred through prescribed fluid overload. On the other
hand, management of severe cases can be difficult, and should be conducted wand electrolytes imbalance.
Summary
Induction of ovulation is an art, as much as being a
science. Even strict vigilance and attention to details will not prevent
complications altogether. Accordingly, doctors involved with this discipline
should have good experience in prescribing the drugs used for induction of
ovulation. They should also be competent in monitoring patients to optimise
response, and to prevent or detect early signs of any complication. It is
always important not to prescribe gonadotrophins if there are no
competent monitoring facilities in place, and there is no hospital backup support
to deal with any complication which may result from such medication.
References
1. Carmina E and
Lobo R A. Polycystic ovary syndrome (PCOS): Arguably the most common
endocrinopathy is associated with significant morbidity in women. J Clin
Endocrinol Metab 1999; 84(6): 1897-1899.
2. Abdel Gadir,
A., Khatim MS. Mowafi RS, Alnaser HM, Muharib NS and Shaw RW. Implications of ultrasonically diagnosed polycystic
ovaries (1)-Correlation with basal hormonal profiles. Human Reproduction 1992;
7 (4), 453-457
3. Greenblat RB.
Chemical induction of ovulation. Fertil Steril 1961; 12: 402-404.
4. Product
monograph. Clomiphene citrate. Ovulatory agent. Submission control number
105030. S-A Version 1.1 July 7, 2008
5. Fluker MR, Wang IY, Rowe TCAn extended 10-day course of
clomiphene citrate (CC) in women with CC-resistant ovulatory disorders. Fertil
Steril 1996; 65(%): 761 - 764.
6. Hughes E, Collins J and
Vanderkerckhove P. Clomiphene citrate for unexplained subfertility in women.
Cochrane Database Syst Rev 1:CD000057.
7. Palomba S. Russo T, Orio Jr F,
Falbo A, Manguso F, Sammartino A, Tolino A, Colao A, Carmina E and Zullo F.
Uterine effects of clomiphene citrate in women with polycystic ovary syndrome:
a prospective controlled study. Hum Reprod 2006; 21(11): 2823 - 2829.
8. Qublan H, Amarin Z, Nawasreh M,
Diab F, Malkawi S, Al-Ahmad N, and Balawneh M. Luteinised unruptured follicle
syndrome: incidence and recurrence rate in infertile women with unexplained
infertility undergoing intrauterine insemination. Hum Rep 2006; 21(8): 2110 –
2113.
9. Abdel Gadir, A., et al., (1985)
Combined clomiphene citrate and oestradiol benzoate therapy in hyperandrogenic
clomiphene citrate non-responding women. Journal of Kuwait Medical Association,
19, 109 - 114.
10. Elnashar A, Abdelmageed E, Fayed M and Sharaf M.
Clomiphene citrate and dexamethasone in treatment of clomiphene
citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled
study. Hum Reprod 2006; 21(7): 1805 - 1808.
11.Brown J, Farquhar C, Beck, Boothroyd C and Hughes
H. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane
Database Syst Rev 2009; 4: CD002249.
12.Eckmann KR and Kockler Dr. Aromatase inhibitors for
ovulation and pregnancy in polycystic ovary syndrome. Ann Pharma cother 2009;
43(7): 1338 - 1346.
13.Goldenberg N, Glueck CJ, Loftspring M, Sherman A
and Wang P. Metformin-diet benefits in women with polycystic ovary syndrome in
the bottom and top quintiles for insulin resistance. Metabolism 2005; 54: 113 -
121
14.Tan S, Hahn S, Benson S, Dietz T, Lahner H, Moeller LC, Schmidt M,
Elsenbruch S, Kimmig R, Mann K and Janssen OE. Metformin improves polycystic ovary syndrome
symptoms irrespective of pre-treatment insulin
resistance. Eur J
Endocrinol 2007; 157:
669 – 676.
15.Siebert TI, Kruger TF, Steyn DW and Nosarka S. Is addition of metformin efficacious in
the treatment of clomiphene citrate-resistant patients with polycystic ovary
syndrome? A structured literature review. Fertil Steril 2006; 86(5): 1432 –
1437.
16.Glueck CJ, Phillips H, Cameron D, Sieve-Smith L and
Wang P. Continuing metformin throughout pregnancy in women with polycystic
ovary syndrome appears to safely reduce first-trimester spontaneous abortion: a
pilot study. Fertil Steril 2001; 75(1): 46 - 52.
17. Khattab S, Mohsen IA, Foutouh IA, Ramadan A, Moaz
M and Al-Inany H. Metformin reduces abortion in pregnant women with polycystic
ovary syndrome. Gynecol Endocrinol 2006; 22(12): 680 - 684.
18. Palomba S. Russo T, Orio Jr F, Falbo A, Manguso F,
Cascella T, Tolino A, Carmina E Colao A, and Zullo F. Uterine effect of
metformin administration in anovulatory women with polycystic ovary syndrome.
Hum Reprod 2006; 21(2): 457 - 465.
19.Glueck CJ,
Wang P, Kobayashi S, Phillips H and Sieve-Smith L. Metformin therapy throughout
pregnancy reduces the development of gestational diabetes in women with
polycystic ovary syndrome. Fertil Steril 2002; 77(3): 520 - 525.
20.Glueck CJ,
Wang P, Goldenberg N and Sieve-Smith L. Pregnancy outcome among women with
polycystic ovary syndrome with metformin. Hum Reprod 2002; 17(11): 2858 - 2864.
21.Bayram N, van
Wely M and van der Veen F. Recombinant FSH versus urinary gonadotrophins or
recombinant FSH for ovulation induction in subfertility associated with
polycystic ovary syndrome (Cochrane Review). In The Cochrane Library, 2003
Issue 1. Oxford Update Software.
22.Daya S and
Gunby J. Recombinant versus urinary follicle stimulating hormone for ovarian
stimulation in assisted reproduction cycles (Cochrane Review). In the Cochrane
Library, 2003 Issue 1. Oxford Update Software.
23.Abate A,
Nazzaro A, Salerno A, Marzano F, Rosaria M, Cossut P and Perino M. Efficacy of
recombinant versus human derived follicle stimulating hormone on the oocyte and
embryo quality in IVF-ICSI cycles: Randomised, controlled, multicentre trial.
Gynecol Endocrinol 2009; 25 (8): 479 – 484.
24.Farhi J,
Orvieto R, Gavish O and Homburg R. The association between follicular size on
human chorionic gonadotropin day and pregnancy rate in clomiphene citrate
treated polycystic ovary syndrome patients. Gynecol Endocrinol 2010; 26(7): 546
– 548.
25.Kupesic S,
Bekavac I, Bjelos D and Kurjak A. Assessment of endometrial receptivity by
transvaginal colour Doppler and three dimensional power Doppler ultrasonography
in patients undergoing in vitro fertilisation procedures. J Ultrasound Med
2001; 20(2): 125 – 134.
26.Goswami RK and
Steptoe PR. Doppler ultrasound studies of the uterine artery in spontaneous
ovarian cycles. Hum Reprod 1988; 3: 721 – 726.
27.Goswami RK,
Williams G and Steptoe C. Decreased uterine perfusion: A cause of infertility.
Hum Reprod 1988; 3: 955 – 959.
28.Filicori M,
Cognigni GE, Gamberini E, Parmegiani L, Troilo E and Roset B. Efficacy of
low-dose human chorionic gonadotropin alone to complete controlled ovarian
stimulation. Fertil Steril 2005; 84(2): 394 -401.
29.Abdel Gadir A,
Khatim MS, Alnaser HM, Mowafi RS and Shaw RW. Ovarian electrocautery: Responders versus non-responders.
Gynecol Endocrinol 1993; 7: 43 - 48.
30.Amer
SA, Li TC and Ledger WL. The value of measuring antimullerian hormone in women
with anovulatory polycystic ovary syndrome undergoing laparoscopic ovarian
diathermy. Hum Reprod 2009; 24(11): 2760 – 2766.
31.Abdel
Gadir A. Ovarian surgery. In: The control and stimulation of follicular growth.
Advances in Reproductive Endocrinology. Edited by RW Shaw, volume 5, pp 111 -
124. The Parthenon Publishing Group, Carnforth, Lancs. 1993.
32.Weerakiet
S, Lertvikool S, Tingthanatikul Y, Wansumrith S, Leelaphiwat S and Jultanmas R.
Ovarian reserve in women with polycystic ovarian syndrome who underwent
laparoscopic ovarian drilling. Gynecol Endocrinol 2007; 23(8): 455 – 460.
33.Murat A. Is
ovarian reserve diminished after laparoscopic ovarian drilling? Gynecol
Endocrinol 2009; 25(3): 159 – 165.
34.Tsafrir A, Simon
A, Margalioth EJ and Laufer N. What should be the first-line treatment for
unexplained infertility in women over 40 years of age - ovulation induction and
IUI, or IVF. Reprod Biomed Online 2009; 19 Suppl 4: 4334.
35.Navot D, Bergh PA
and Laufer N. The ovarian hyperstimulation syndrome. In: Adashi EY, Rock JA and
Rosenwaks Z. (eds.) Reproductive Endocrinology, Surgery and Technology.
Philadelphia. Lippincott-Raven. 1996. pp. 2215 – 2232.
36.The Practice
Committee of the American Society for Reproductive Medicine. Ovarian
hyperstimulation syndrome. Fertil Steril 2004; 82(1): S81 – S86.
37.Kasum M. Ovarian
hyperstimulation syndrome. Gynecol Perinatol 2004; 13(2): 62 – 68.
38.Mayorga MP,
Gromoll J. Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to
follicle stimulating hormone (FSH) stimulation depends on FSH receptor
genotype. J Clin Endocr Metab 2000; 85: 3365 – 3369.
39.Brinsden P, Wada
I, Tan SL, Balen A and Jacobs HS. Diagnosis, prevention and management of
ovarian hyperstimulation syndrome. Br J Obstet Gynaecol 1995; 102(10): 767 –
772.
40.Asch RH, Li H,
Balmaceda JP, Weckstein LN and Stone SC. Severe hyperstimulation syndrome in
assisted reproductive technology: definition of high risk groups. Hum Reprod
1991; 6: 1395 – 1399.
41.Levy T, Orvieto
R, Homburg R, Peleg D, Dekel A and Ben-Rafael Z. Severe ovarian
hyperstimulation syndrome despite low plasma oestrogen concentration in a
hypogonadotrophic hypogonadal patient. Hum Reprod 1996; 11(6): 1177 – 1179.
42.Morris RS.
Paulson RH and Herman A. Serum oestradiol concentration and ovarian
hyperstimulation syndrome. Hum Reprod 1995; 9: 811 – 814.
43.Blankstein J,
Shalev J, Saadon T, Kukia EE, Rabinovici J, Pariente C, Lunenfeld B, Serr DM
and Mashiach S. Ovarian hyperstimulation syndrome: prediction by number and
size of preovulatory ovarian follicles. Fertil Steril 1987; 47(4): 597 – 602.
44. Abramov Y, Barak
V, Nisman B, Schenker JG. Vascular endothelial growth factor as capillary
permeability agent in ovarian hyperstimulation syndrome. Lancet 1994; 344: 235
– 238.
45.Wang Q, Yang K,
Yang C, Zhan B, Yang R. The predictive role of vascular endothelial growth
factor and oestradiol in infertile patients with ovarian hyperstimulation
syndrome. Sichaun Da Xue Xue Bao Yi Xue Ban 2003; 34; 565 – 568.
46. McElhinney B,
Ardill J, Caldwell C, Lloyd F, McClure N. Variations in serum vascular
endothelial growth factor binding profiles and the development of ovarian
hyperstimulation syndrome. Fertil Steril 2002, 78: 286 – 290.
47. McElhinney B,
Ardill J, Caldwell C, Lloyd F, McClure N. Ovarian hyperstimulation syndrome and
assisted reproductive technologies: why some and not other? Hum Reprod 2002;
17: 1548 – 1553.
48.Rabau E, Serr DM,
David A, Mashiach S and Lunenfeld B. Human menopausal gonadotrophin for
anovulation and sterility. Am J Obstet Gynecol 1967; 98: 92 – 98.





